Egnyte Life Sciences
Simple to use, easy to deploy, and built to perform under GxP regulatory standards.

Trusted by Over 600 Life Science Companies Worldwide
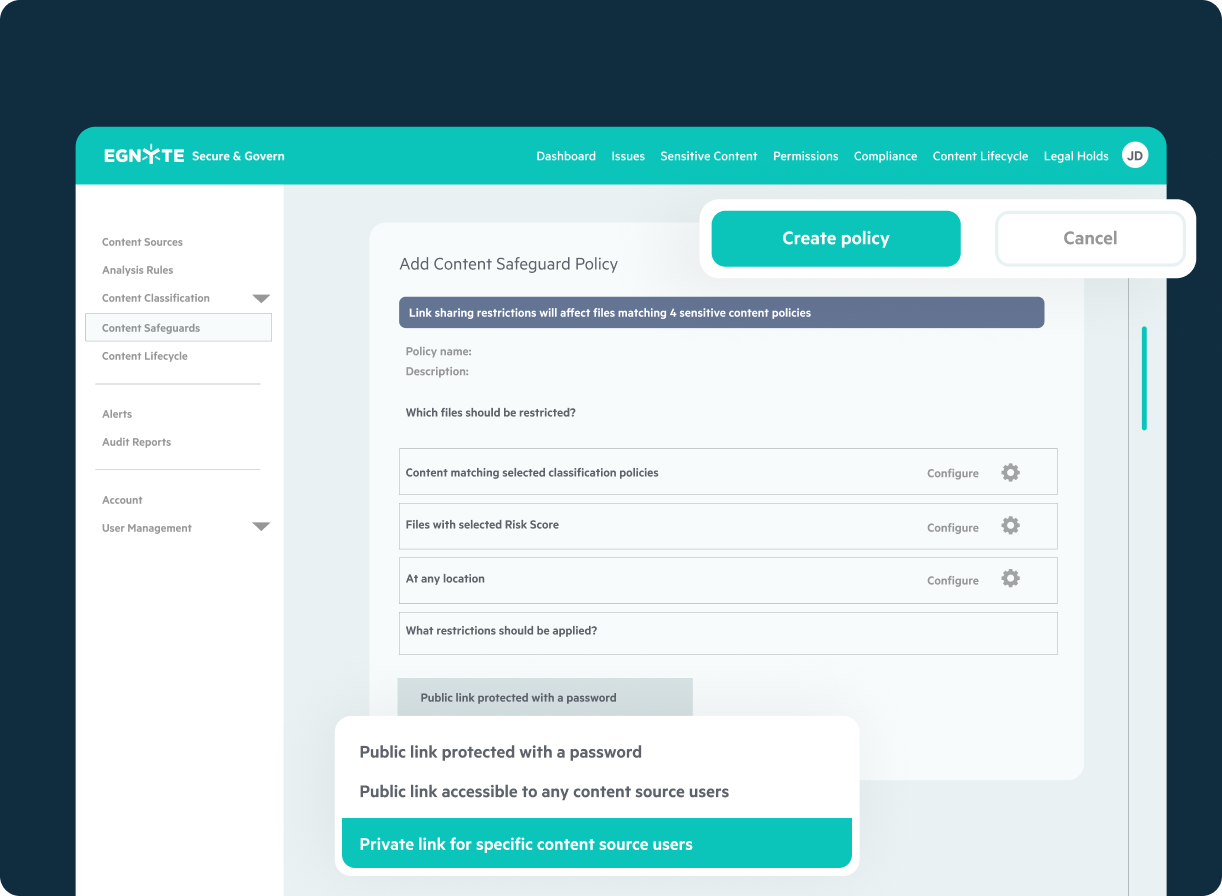
Unified Document Management and Control
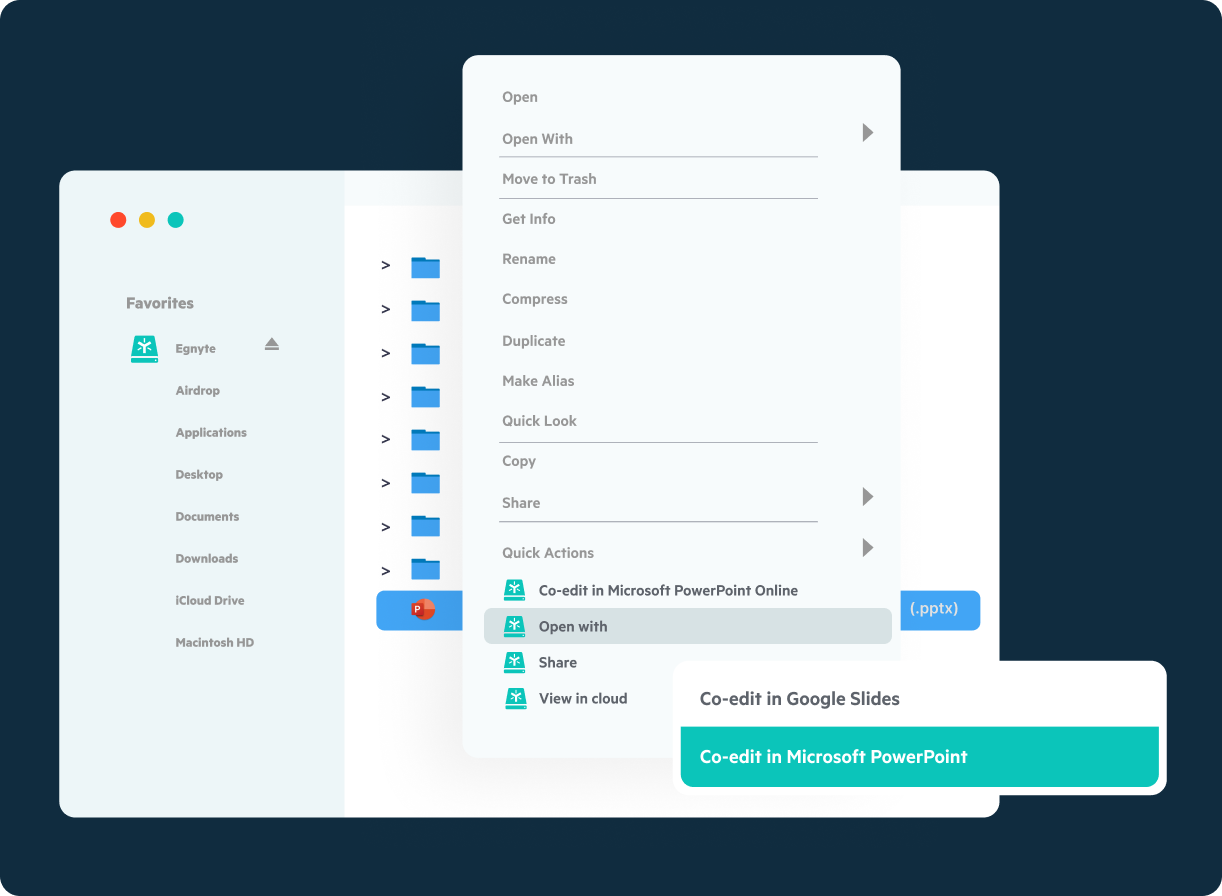
Collaboration
Securely share files with CROs, sites and partners and collaborate intuitively using native co-editing in Microsoft and Google Workspace. Organize and maintain intellectual property, financials and key company data for sharing with investors and partners.
Lab Data Management
The easiest way to collect, transfer and manage large volumes of lab instrument data and workflows to the cloud while maintaining data integrity and security.
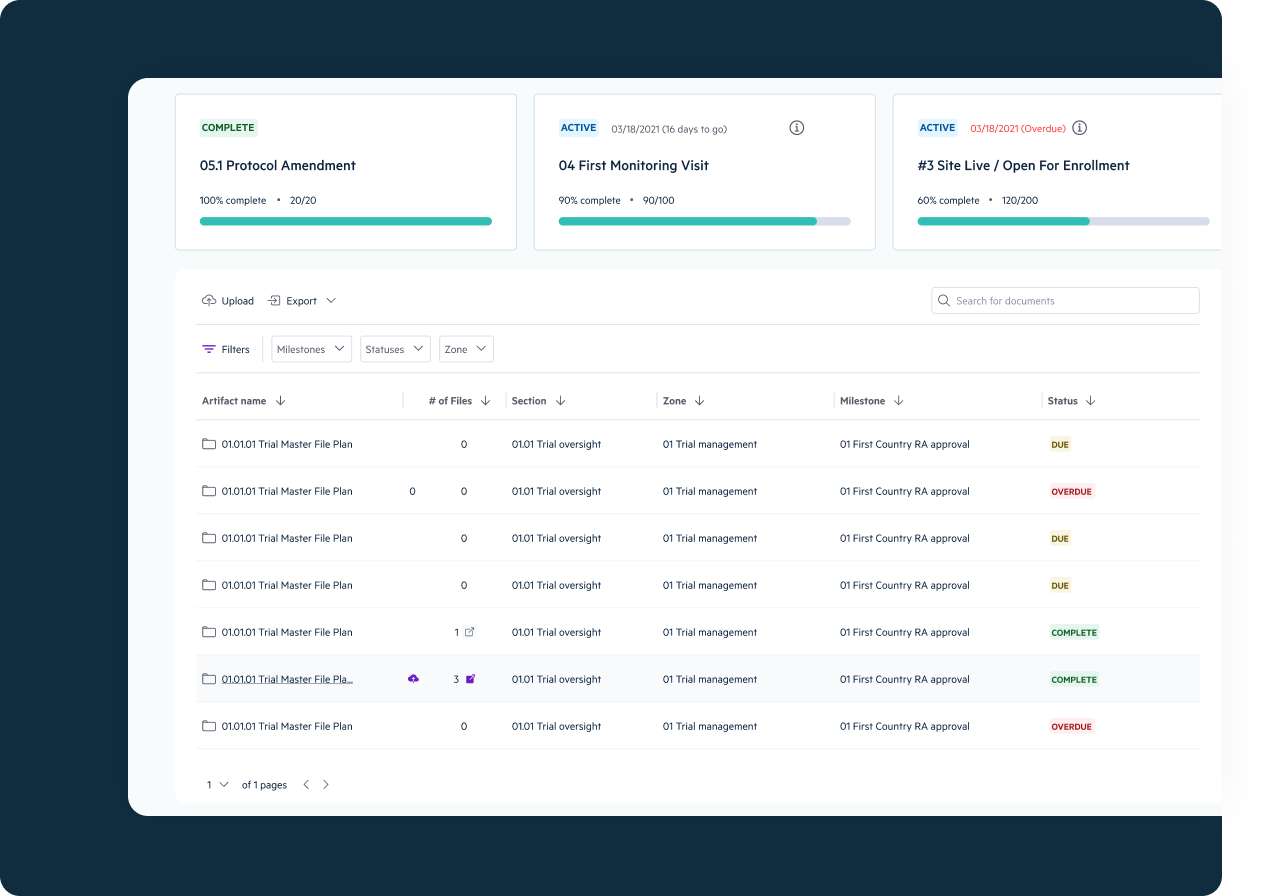
eTMF Management
Assemble all critical trial documents in a cloud-based document management system that grants visibility into trial completeness, quality, and timelines, with built-in audit-readiness for regulatory review.
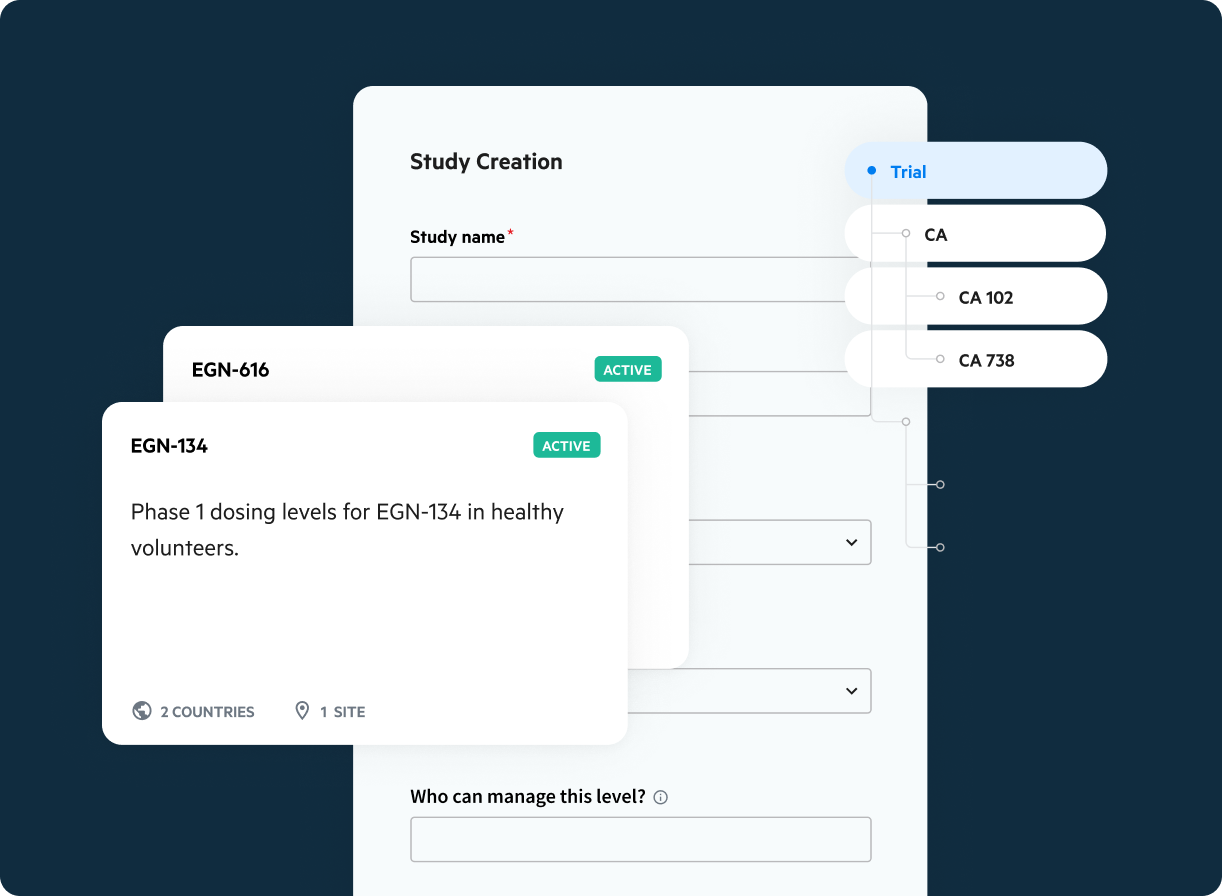
Statistical Computing Environment
Simplify your statistical data analysis workflow using a centralized computing environment for research and development that integrates all of your data, metadata, programs, and results.
Plans For Life Science Companies Of All Sizes
Business
- Multi-device collaboration
- Co-editing
- Privilege management
- Ransomware protection
Egnyte for Life Sciences
- Multi-step workflows
- Controlled document management
- Audit reports with file checksums
- Threat detection
Egnyte for Life Sciences, GxP
- GxP compliance dashboard
- Validation as a service
- Production qualification reports
- Content lifecycle analytics and reporting
Popular Add-Ons for Life Sciences
Instrument Service AccountsEnsure lab generated data is transferred to the cloud with full audit trails.
Ensure lab generated data is transferred to the cloud with full audit trails.
eTMFSet up, manage, track and report on the progress of trial documentation.
Set up, manage, track and report on the progress of trial documentation.
Learning and Training ManagementKeep your teams compliant with latest SOPs and work instructions.
Keep your teams compliant with latest SOPs and work instructions.
Trusted by Leading Life Science Companies
Founders
Learn how Third Rock Ventures deploys Egnyte’s simple, secure data management and collaboration platform into every biotech start-up’s tech stack.

Research
Learn how Nimbus Therapeutics uses Egnyte to automate CRO data ingestion to accelerate research deployment to scientists.

Clinical
Learn how Vial accelerates workflows, reduces costs and improves partner collaboration with Egnyte’s eTMF solution.

IT
Learn how Revolution Medicines uses Egnyte to deliver compliant audit data submissions for their clients.

Additional Life Sciences Resources

Transforming Collaboration and Data Management

How to Improve Collaboration with CROs and Sponsors
