SECURE ENCLAVE SOLUTION
GxP Compliance
GxP Compliance for Emerging Biotechs
From managing regulated documents to ensuring data integrity, Egnyte is the easiest way for emerging biotechs to get over the compliance hurdle. Effective and efficient clinical data management gets you to trial completion faster.
Speed to Patient
Achieve rapid validation without slowing down the pace of innovation.
Unified Compliance
Facilitate management of regulatory and unregulated content with a single, centralized data repository.
Optimized Workflows
Increase process efficiency through automated review and approval workflows.
GxP Compliance Made Easy
21 CFR Part 11 Compliant
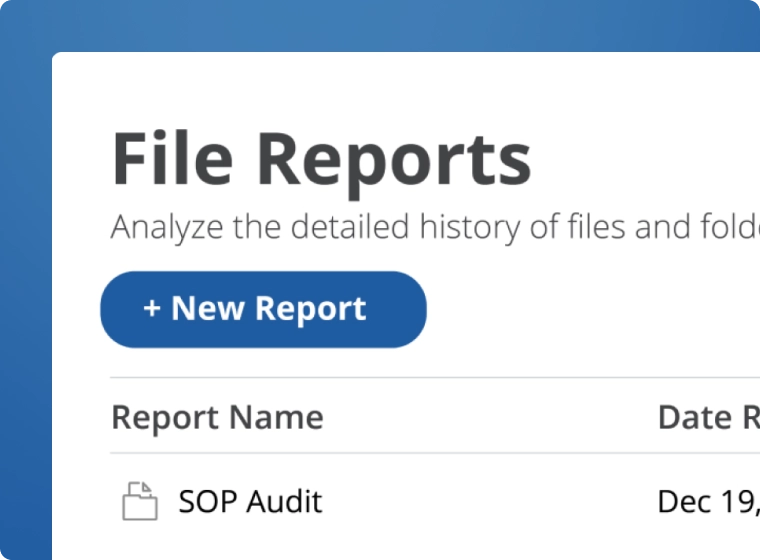
Comply with regulatory requirements when creating, storing, and managing GxP-regulated documents. Native support for audit trails, checksums for data integrity, and robust access control deliver a simple yet effective path to GxP and 21 CFR Part 11 compliance.
Easy to Implement & Manage
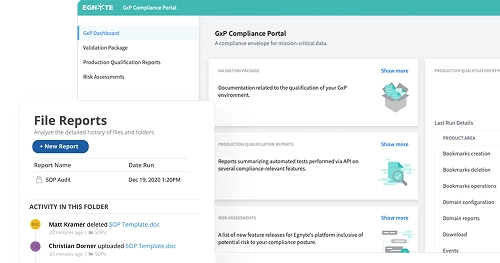
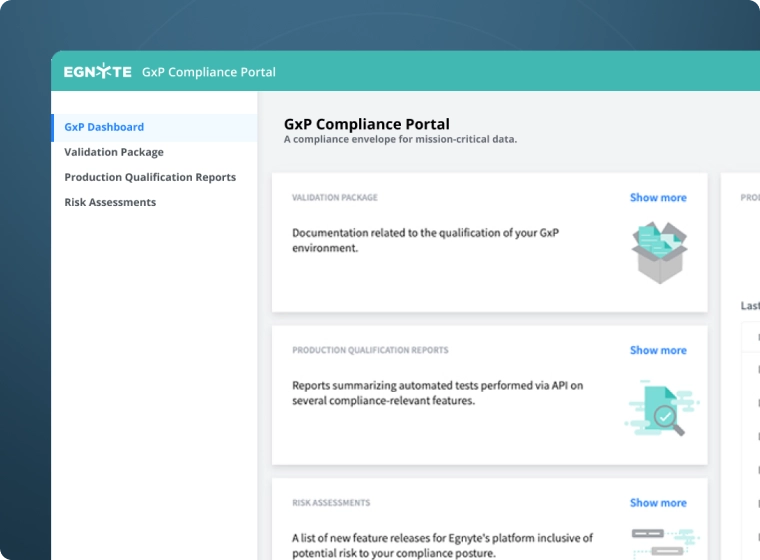
Establish a regulated environment accessible only to credentialed employees and external partners, whether your compliance envelope covers all your documents or just a single type. Use the compliance portal as a central hub to facilitate auditing, validation, and reporting.
Ramp Up Your Controlled Documentation Process
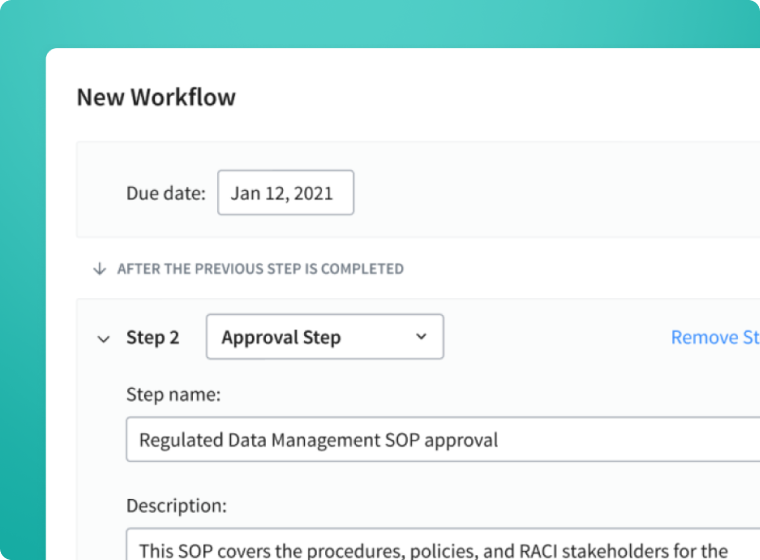
Enable an effective document management process by facilitating collaboration, complying with regulatory requirements, and reducing the administrative burden of maintaining controlled documents.
The Power of Many Solutions In One Turnkey Platform
GxP Enterprise Lite
- GxP Compliance Portal
- Audit Trails
- Multi-Step Workflows
- Data Lifecycle Management
GxP Enterprise
- GxP Compliance Portal
- Audit Trails
- Rapid Validation
- Content Safeguards
- Ransomware Detection & Recovery
Over 600 Life Sciences Customers Trust Egnyte
See Egnyte GxP in Action
Egnyte for Life Sciences Streamlines GxP Compliance
- Validate systems with Part 11-compliant documents
- Automate regulated document management with multi-step review and approval
- Maintain audit-readiness with file versioning and audit trail reporting


