Trusted by Over 600 Life Science Companies Worldwide


Solutions for data governance, compliance, and collaboration in clinical research.
Plans for Emerging and Established Life Sciences Companies
Egnyte for Life Sciences Standard
- Securely share and collaborate via cloud, mobile, and desktop apps
- Microsoft and Google co-editing
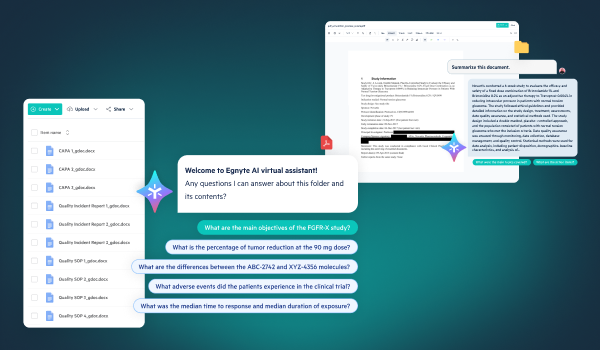
- AI-powered document summary and Q&A
- Life Sciences workflows
- Probable ransomware detection
Egnyte for Life Sciences Professional
- Watermarking
- Document type classification
- Content safeguards
- Privacy and compliance monitoring
- Advanced snapshot and recovery
Egnyte for Life Sciences GxP with Governance
- GxP Validation
- Life Sciences quality and quality training
- Life Sciences workflows
- Securely share and collaborate via cloud, mobile, and desktop apps
- Document type classification
- Probable ransomware detection
Plans for Emerging and Established Life Sciences Companies
Trusted by Leading Life Science Companies


Research and Development
Revolution Medicines Improves Collaboration Across All Aspects of Research and Development


Additional Life Sciences Resources
FAQs
Managing regulated data in the life sciences industry requires a secure, compliant, and scalable platform that ensures traceability of data across its lifecycle. The best solutions offer centralized data access, role-based permissions, and built-in support for FDA 21 CFR Part 11, Annex 11, GDPR, HIPAA and other regulatory standards. Validated platforms like Egnyte are commonly used to ensure the integrity, reliability, quality and security of both structured and unstructured data assets.
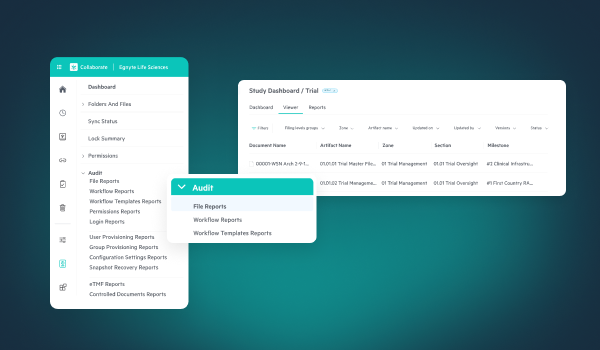
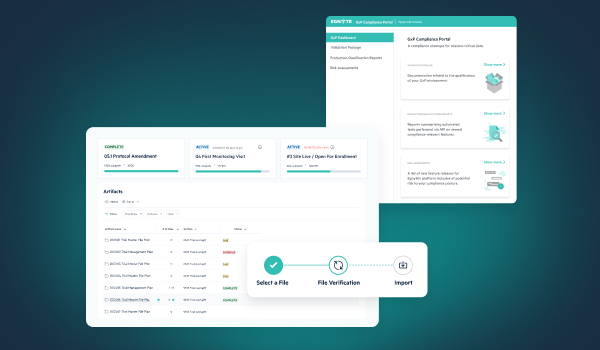
Ensuring a technology solution is compliant begins with understanding the intended use of the system and ensuring it is validated for that use. Solutions like Egnyte help streamline compliance by aligning platform requirements to regulatory requirements such as easy to access and comprehend audit trail reports and 21 CFR Part 11 compliant electronic signatures, as well as ensuring the platform is in a continuously validated state with inspection ready documentation available to all GxP customers.
Life sciences organizations manage unstructured data—like lab reports, images, and research documents—by using content management systems that provide scalable storage, advanced search, metadata tagging, and lifecycle management. Egnyte offers intelligent data governance and streamlined access to unstructured data for R&D, clinical, and regulatory teams.
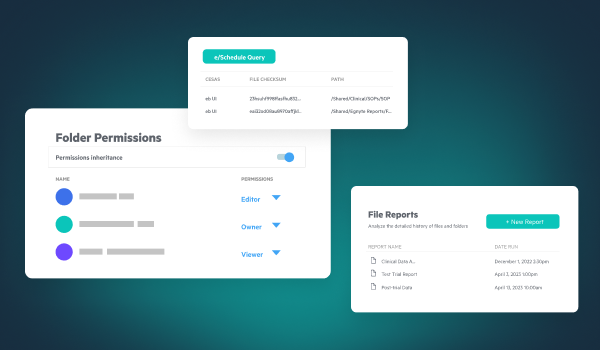
Data security is critical in life sciences to protect sensitive research, intellectual property, clinical trial data, and patient information. Breaches can lead to regulatory fines and loss of trust. Secure platforms with encryption, access control, and real-time monitoring help safeguard data throughout the R&D lifecycle.
Cloud solutions enhance collaboration by enabling secure real-time access to research data, protocols, and regulatory documents across distributed teams. This improves efficiency, accelerates development timelines, and ensures that partners, CROs, and external stakeholders can easily and securely contribute to projects.
Key features to look for include regulatory compliance tools, secure data sharing, document version control, audit trails, automated workflows, and integrations with lab and clinical systems. A platform like Egnyte is tailored to meet the specific needs of biotech, pharma, and medical device companies.