Trusted By Life Sciences Companies
Egnyte’s Unified Platform Grows With You at Every Stage of The Drug Development Lifecycle
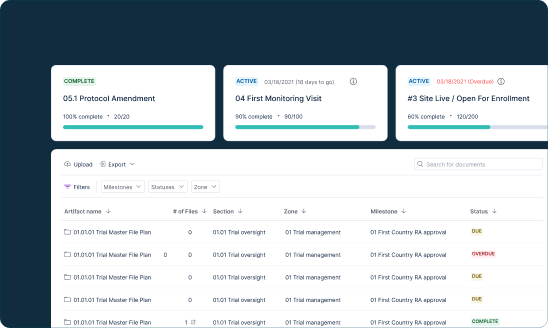
Simplify Clinical Data Management Across Your Partner Ecosystem
- Collaborate securely across your ecosystem of CRO’s and partners
- Control permissions across users, roles, and organizations with support for a variety of regulated and non-regulated content
- Increase productivity with built-in document review and approval workflows


Manage Your Content Lifecycle of All Types of Documentation
- Seamlessly integrate with business and scientific applications like Microsoft Office, Google Workspace and AWS
- Automate collaboration on large datasets like genomics and images
- Achieve real-time access to content via cloud only, multi-cloud, and hybrid options to meet every business process
Accelerate Time to Patient, GxP Validation, and 21 CFR Part 11 Compliance**
- Seamlessly consolidate all trial documentation across multiple study sites
- Leverage comprehensive audit trails to get full visibility into trial completeness, quality, and timeliness
- Simple QC workflows and built-in metrics to ensure accurate filing within eTMF
**Available with the expanded Life Sciences solution

PC Mag
“Egnyte is one of the strongest offerings on the market.”

Gartner Peer Insights
“Gartner Peer Insights Customers’ Choice Winner for Content Collaboration Platforms”

G2
“A Leader in File Storage and Sharing for 2024”

Forrester
“390% Average Return on Investment (ROI) When Deploying Egnyte”
Speak With an Egnyte Life Sciences Specialist Today
Chat NowEGNYTE FOR LIFE SCIENCE
21 CFR Part 11
Comply with regulatory requirements to manage your electronic records and electronic signatures. Native support for audit trails, checksums for data integrity, and robust access control deliver a simple yet effective path to GxP and 21 CFR Part 11 compliance.
EGNYTE FOR LIFE SCIENCE
eTMF
Assemble all critical trial documents in a cloud-based document management system that grants visibility into trial completeness, quality, and timelines, with built-in audit-readiness for regulatory review. Reduce the time spent on administration of site documentation, searching for and organizing documents, and submission preparation.
EGNYTE FOR LIFE SCIENCE
Clinical Data Management
Effectively manage regulated data to achieve operational & scientific excellence. Ensure data quality and integrity with the easy classification of regulated and unregulated data, automated policies for retention, deletion, and archival, multi-party workflows that support visibility, data quality, and integrity, and co-editing capabilities to accelerate the review and approval process.


