How to Select an Electronic Laboratory Notebook
The use of electronic laboratory notebooks is growing as labs seek ways to meet demands to deliver results faster while reducing errors. Because of its automation and other tools, an electronic laboratory notebook allows for the collection and organization of data more quickly and reliably than paper lab notebooks.

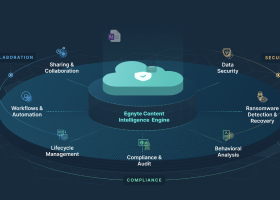
Distributed access, sharing, collaboration, and analysis capabilities also increase productivity and expedite time to results. In addition, an electronic laboratory notebook platform aggregates information about experiments, making it easily searchable and reusable for reporting and subsequent experiments.
Electronic laboratory notebooks also address another challenge facing labs—reproducibility. This is important not just for the accuracy and efficacy of research, but to meet compliance requirements.
Multiple factors negatively impact reproducibility. These can be mitigated with electronic laboratory notebooks, which help researchers more consistently and correctly document experimental data improving reproducibility.
Electronic Laboratory Notebooks and FDA 21 CFR Part 11
Here are some ways that 21 CFR Part 11 compliant electronic laboratory notebooks help meet regulatory requirements:
- Data collected and maintained in the electronic laboratory notebooks may be submitted to the FDA and can be used to satisfy record approval requirements.
- Organizations can be sure that data is secure and adheres to requirements for privacy.
- A built-in audit trail system satisfies the data trustworthiness requirement.
- All electronic signatures are securely generated.
- System access is controlled, and any attempts at unauthorized access are promptly detected.
The ELN Market
Several drivers for electronic laboratory notebooks (ELNs) replacing paper laboratory notebooks are related to data recording, sharing, and security. In addition, an increasing need to reduce costs, improve workflow speed, more efficiently capture and manage documentation, and a desire for data to support analytics are fueling the adoption rate for electronic laboratory notebooks.
Expansive and growing compliance requirements are also driving the ELN market. Electronic laboratory notebooks support compliance with GxP guidelines, FDA 21 CFR Part 11, HIPAA, and other regulatory requirements.
Categories of Electronic Laboratory Notebooks
- Cross-disciplinary
- Specific
- Cloud-based / web-hosted
- Open-source
Industries Using Electronic Lab Notebooks
Electronic laboratory notebooks are used in a growing number of markets due to their track record of improving product quality, helping with product development, and enhancing operational efficiencies. Among the industries that have significant adoption of electronic laboratory notebooks are:
- Life sciences
- Agriculture
- Chemical industry
- Clinical research organizations (CROs)
- Environmental testing labs
- Food and beverage
- Forensics
- Metal and mining labs
- Oil and gas
- Petrochemical refineries
Benefits of ELNs
Among the many benefits of ELNs are:
- Ability to add, select, tag, track, and share files, external links, and graphs internally and externally
- Advanced search functionality
- Audit trails for compliance requirements
- Consolidated, centralized data management with all information collected and stored in the same place
- Creation of manuscripts by selecting digital research notes, experiments, protocols, images, references, and results
- Ease of tracking sample information
- Enhanced collaboration with a more accessible, digitized way to work on experiments and projects
- Primary legal document that is acceptable for regulatory, patent, and intellectual property matters
- Share ELN data in and outside the lab for current and future research
- Standardization that supports reproducibility of tests and experiments
- Tight access controls for data integrity and security
Disadvantages of ELNs
Most organizations have found the benefits of ELN far outweigh the potential risks. However, a few disadvantages of ELNs to note are:
- Challenges related to the conversion process
- Cost of licenses and implementation
- Potential damage to the device holding the ELN in the lab
- An evolving market
- Risk of a security breach
Steps to Selecting an ELN
Electronic laboratory notebooks have tremendous potential. To ensure that the right system is selected, it is important to understand the features and functions as well as the full scope of engagement required to bring them online effectively.
These steps to evaluating and selecting an ELN can help ensure that the selected system not only meets requirements, but can be successfully deployed with available resources.
Electronic Laboratory Notebook Capabilities to Consider
- Ability to create reports based on custom parameters or automatically create pre-configured reports
- Advanced search that provides a comprehensive display of results, making it easy to find data
- Inventory management to create lists of items and connect them to experiments
- Notes integrated into the system and automatically placed in the proper position within the data structure
- Orchestration of the design, execution, analysis, and reporting of experiments
- Ability to easily generate printed materials
- Support for multiple file types to allow users to import different data formats
Electronic Laboratory Notebook Functionality to Consider
- Audit trails include automated time stamping of actions, such as written comments, added files, and notes
- Backup data storage scheduling
- Cloud or on-premise deployment
- Collaboration capabilities include comments, notifications, sharing files, and delegating tasks
- Compliance and certification requirements met to adhere to FDA 21 CFR Part 11, GLP, and ISO
- Data access is controlled and protected by specific permissions according to person, labs, and third parties
- Number of users both in terms of system capacity and licensing fees
- Overall ease of use—does not require complex integration or intensive training
- Security measures to ensure data integrity and protect from unauthorized access or theft
- Technical support team
Electronic Laboratory Notebook Evaluation Best Practices
- Get the right people from across the organization involved early:
- Include lab managers, lab assistants, scientists, virtual research teams, external partners, Contract Research Organizations (CROs), regulatory personnel, IP specialists, legal, and IT.
- Ensure that the electronic laboratory notebook meets the needs of the organization as a whole.
- Involve departments that may wish to use an electronic laboratory notebook in the future.
- Assess requirements carefully by asking questions:
- Who are the users?
- What are current lab notebook workflows?
- What other systems need to be integrated?
- What are the geographic usage requirements—i.e., local, global?
- How should the system be deployed—i. e., cloud-based, on-premises system?
- Select vendors for consideration:
- Base selection on the ability to meet core requirements.
- Share the results of the requirements assessment.
- Invite vendors to visit labs, meet scientists, and understand work processes.
- Be an active participant in product demonstrations by developing use cases that exemplify requirements in detail:
- Ask vendors to demonstrate how their solution works with use cases.
- Understand the level of effort it took to support these use cases.
- Clarify which functionality is supported out-of-the-box and which requires customization.
- Learn about timing and costs for any customization.
- Understand the total cost of ownership.
- Dig into reference accounts to get clarification and confirmation about the information gathered to date, as well as anything that was not covered:
- Were there any hidden costs?
- How was the implementation process, and did it proceed according to schedule?
- How is the performance of the system in production?
- Were there any issues post-implementation?
- Would they recommend the vendor?
- Did the vendor have resources readily available for issue resolution and customizations?
- Pilot the system to ensure that it meets the expectations and requirements of users.
A Strategic and Competitive Edge
The move to electronic laboratory notebooks is considered to be quite an easy transition, as user interfaces have been optimized for specific use cases. Users find that electronic laboratory notebooks are easier to use, because of the robust features that are included in the system.
Management benefits by having work consolidated in a single system that can be accessed across users and teams. And the return on investment proves to be significant as electronic laboratory notebooks increase productivity, improve reproducibility, and enhance overall efficiency.
Implementing electronic laboratory notebooks can also provide a strategic and competitive edge by up-leveling scientific information capture, workflow optimization, process consistency, IP protection, collaboration, and knowledge sharing.
Egnyte has experts ready to answer your questions. For more than a decade, Egnyte has helped more than 16,000 customers with millions of customers worldwide.
Last Updated: 23rd October, 2021