What is a Clinical Trial Management System (CTMS)?
A clinical trial management system (CTMS) is a software system used to manage all of the activities related to the setup, conduction, and closeout of clinical trials, including planning, preparation, tracking, monitoring, compliance, and reporting. Many clinical trial management systems are web-based, making them easy to use across multiple sites with a large group of users.

The clinical trial management system is the system of record for study data, which can be shared with other systems according to the study protocol. Often, clinical trial management systems share data with a business intelligence (BI) system, which acts as a digital dashboard for clinical trial managers. By centralizing data and automating processes, a clinical trial management system makes clinical trial information more accessible and transparent.
Why a CTMS is Important
A CTMS can be used for all four phases of a clinical trial. Research sites, institutions, sponsors, and contract research organizations (CROs) use clinical trial management systems to ensure studies' success.
Some of the many reasons a CTMS is important are that it saves time and reduces frustration over the course of a study by:
- Providing ready access to an overview of the progress of a study with continuous and up-to-date reporting
- Allowing study leaders to plan tasks and activities as well as assign responsibilities, and track and monitor activities
- Organizing all of the study’s relevant documents and information
- Managing a range of different assets and associated clinical trials
- Facilitating compliance with regulatory requirements
Features of a CTMS
The number, size, and scope of clinical trials and their associated operational resources will dictate what features of a CTMS are required. Following are features commonly sought in a clinical trial management system.
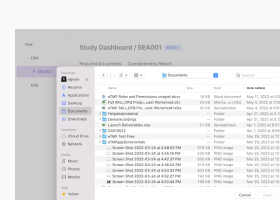
Protocol and Clinical Study Documentation Tracking and Management
- Review, manage, and author essential study documents
- Approve clinical trial documentation
- Track the status of clinical trial documentation throughout its lifecycle
- Create, manage, and protect the electronic Trial Master File (eTMF)

Clinical Trial Project Management
- Enable oversight and management of clinical trials based on project activities, timelines, and resources
- Track progress at specific trial and program levels
- Communicate with the team
- Manage and track submissions
- Coordinate activities among the functional departments
Development and Tracking of Study Forms, Approvals, and Milestones
- Case report forms (CRF) and electronic case report forms (eCF)
- Electronic data capture (EDC) components and summary
- Key clinical trial milestones, such as last patient visit (LPV)
- Regulatory approval tracking, including Institutional Review Boards (IRB) and Independent Ethics Committees (IEC)
Management of Study Financials
- Project and track the budget
- Manage grants
- Pay claims related to study activities, including investigators and other third parties
- File and manage financial disclosures
Site, Subject, and Team Management
- Provide site monitoring and subject enrollment
- Manage site visits and trip reports
- Define and track the roles and responsibilities of all participants in a trial
Study Management and Clinical Monitoring
- Track key information, such as CRF collection, clinical research associate (CRA) monitoring frequency, protocol visit frequency, and adherence to protocol regiment
- Support study documentation and clinical monitoring workflows
- Monitor quality criteria to ensure that the conduct of the trial adheres to regulatory, ethical, and safety standards and requirements
Investigator Management
- Allows the trial sponsor to manage relationships with investigators
- Provides visibility into the status of study data and related status with CROs and other investigator sites
- Tracks approvals by Institutional Review Boards (IRB) and Independent Ethics Committees (IEC)
Issue Management
Manage, track, and resolve issues according to good clinical practice (GCP), such as:
- Protocol deviations
- Study level, country-specific, and site-level issues
- Ensure that ethical, professional, and patient safety principles are adequately maintained
Clinical Supply Management—Storage and Shipment
Oversee and manage:
- Clinical supply logistics for components, such as inventory locations, lots, and shipment details
- Multiple service providers, including CROs, laboratory services, and medical specialists
- Supply tracking
- Clinical supplies
Clinical Trial Data Management
- Archiving
- Warehousing
- Data analysis of trial-generated clinical data
- Query resolution for any discrepancies or inconsistencies found in clinical data
Security
- Monitor the security of the data connected with participants and trial results
- Send notifications about potential security risks
- Provide secure storage for all clinical trial data
- Employ the principle of least privilege for system and data access
- Enforce multi-factor authentication and strong passwords
- Use a SaaS CTMS for access to secure messaging, virus protection, data encryption, and server monitoring
Other Capabilities and Features of a CTMS
- Tracking and management of clinical learning and training requirements required for the clinical trial
- Ability to track and analyze workflows related to the study participants’ reactions
- Reporting and operational metrics
- Optimized for mobile devices
- Payment processing system to track tasks, create receipts, and make payments
- Connection to patients’ wearable devices
Benefits of a CTMS
- Ensures compliance with all legal protocols, guidelines, and standards
- Generates detailed reports
- Identifies potential problems early
- Improves the transparency
- Increases data management efficiency
- Makes it easier to deliver the information in a secure, legal way
- Monitors trials’ progress
- Offers a secure, centralized location for data collection, storage, and retrieval
- Prevents enrollment in multiple trials
- Provides a secure location for all data
Choosing the Right CTMS
There are many considerations when choosing the right CTMS. Following are several items to include on an evaluation scorecard for a clinical trial management system.
Collaboration
- Ability to access the clinical trial management system from every site
- Web-based CTMS for easy access to systems and data
Compliance
- Meets all requirements imposed by HIPAA, GCP, GDPR, 21 CFR Part 11, and other regulations
- Able to adapt to changes in compliance requirements
Patient Recruitment
- Maintain recruitment and patient information in one centralized location
- Able to filter, create calls lists, and track recruitment campaigns
- Help research sites to find new participants
Financial Management
- Track and manage all study budgets
- Maintain invoices
- Manage payables and receivables
Robust Reporting
- Offer strong reporting capabilities
- Provide the tools needed to run reports in real-time
- Visualize data
The CTMS and Compliance
A clinical trial management system helps organizations adhere to regulatory requirements. With regard to the CTMS and compliance, the three most important regulatory requirements are for:
- General Data Protection Regulation (GDPR)
Enforces data protection and privacy in the European Union and the European Economic Area - Good Clinical Practice (GCP)
Ensures that ethical, professional, and patient safety principles are adequately maintained - Health Insurance Portability and Accountability Act (HIPAA)
Safeguards electronic protected health information (ePHI) and establishes the circumstances in which ePHI can be disclosed - Title 21 of the Code of Federal Regulations Part 11 (21 CFR Part 11)
Defines the criteria under which electronic records and electronic signatures are considered trustworthy, reliable, and equivalent to paper records

CDISC has set numerous requirements on how clinical trial data should be captured, stored, used in analysis, and exchanged. A clinical trial management system automatically formats data according to these standards.
An Advantage for Researchers and Sponsors
A clinical trial management system streamlines study activities and improves the financial health of clinical trials. Data management is simplified, financial transparency is ensured, resources are optimized, and errors are prevented. A CTMS provides the tactical support required to run a trial on a daily basis and strategic capabilities to optimize operations.
Egnyte has experts ready to answer your questions. For more than a decade, Egnyte has helped more than 16,000 customers with millions of customers worldwide.
Last Updated: 11th October, 2021