A Guide to IQ, OQ, and PQ in FDA-Regulated Industries
The FDA regulates medical devices, and an important part of complying with the regulations is meeting manufacturing quality requirements. IQ, OQ, and PQ in FDA-regulated industries provide a framework for reliable performance validation of medical device manufacturing equipment and processes. These tiers of qualification consistently validate all stages of manufacturing that meet applicable requirements and specifications to ensure consumer safety.
Medical Devices Per the FDA:
Medical devices range from simple tongue depressors and bedpans to complex programmable pacemakers and closed-loop artificial pancreas systems. Additionally, medical devices include in vitro diagnostic (IVD) products, such as reagents, test kits, and blood glucose meters. Certain radiation-emitting electronic products that have a medical use or make medical claims are also considered medical devices. Examples of these include diagnostic ultrasound products, x-ray machines, and medical lasers.
What Are IQ, OQ, and PQ?
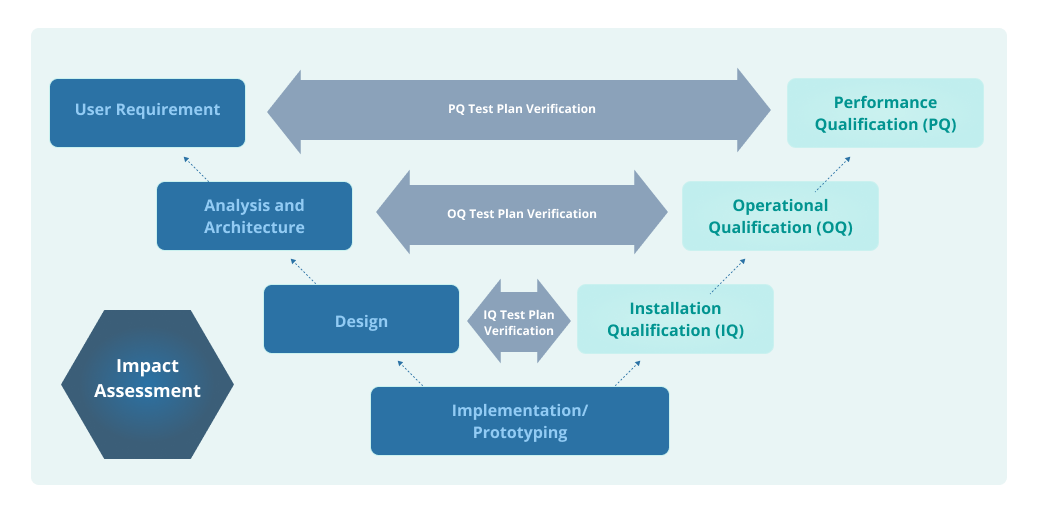
Installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) are vital parts of a quality assurance system. They are the key quality assurance protocols for each phase of pharmaceutical equipment manufacturing.
IQ, OQ, and PQ rigorously determine whether new instruments or equipment are assembled correctly and perform according to the specified requirements. These qualification checks ensure that medical devices deliver consistent results and meet quality standards.
IQ, OQ, and PQ establish documented evidence that production equipment meets quality standards by confirming that:
- Everything is installed correctly—IQ
- Everything is operating correctly, and that operating limits are understood—OQ
- It operates the way in which it is intended under load—PQ
Current good manufacturing practice (cGMP) requirements apply to IQ, OQ, and PQ. Accordingly, the approach employed for conducting the qualifications must be documented in a validation master plan (VMP). Key documents related to IQ, OQ, and PQ included in the VMP are:
- User requirement specification
- Impact assessment
- Risk analysis
- Traceability matrix
- Qualification plans and reports
- Deviation reports
- Final reports
Most pharmaceutical device manufacturers follow the International Society for Pharmaceutical Engineering (ISPE) V-Model to verify and validate compliance as required for FDA-regulated industries.
Installation Qualification (IQ)
IQ is a process that verifies that a medical device has been properly delivered, installed, and configured according to standards set by the manufacturer. The objective of IQ is to validate the medical device manufacturing process to confirm that it is stable and delivers consistent performance. IQ also ensures that variation is minimal and predictable.
IQ verifies and documents that key aspects of an installation meet the approved requirements. These requirements are outlined in a number of documents, including:
- Datasheets
- Design specifications
- Developers’ recommendations
- Manufacturer’s recommendations
- System specifications
During IQ, medical devices are checked against functional specifications developed during the user requirements stage as well as engineering drawings, piping, and instrument diagrams (P&ID) developed during the design phase. IQ processes confirm that everything is installed properly by evaluating many areas related to installation, including:
- Calibration and validation
- Checking proper location
- Components and equipment match the packing list
- Connections with peripherals
- Electrical supply
- Environmental conditions
- Installation
- Records
- Revalidation, facility conditions, and documentation
- Signals and alarms
- Software and systems testing
- Standard operating procedures (SOP)
FDA Requirements for IQ are detailed in 21 CFR 820.70(g)—Production Process Controls/Equipment.
- Ensure that all equipment used in the manufacturing process:
- Meets specified requirements
- Is appropriately designed, constructed, placed, and installed to facilitate maintenance, adjustment, cleaning, and use
- Establish and maintain schedules for the adjustment, cleaning, and other maintenance of equipment
- Conduct periodic inspections to ensure adherence to applicable equipment maintenance schedules
- Post any inherent limitations or allowable tolerances on or near equipment that requires periodic adjustments
- Document maintenance activities, inspections, and any adjustments to equipment, including the date and individual(s) performing these functions
Operational Qualification (OQ)
OQ involves testing the equipment to confirm that it operates as intended, within operating ranges approved by the manufacturer. This process must be performed after installation, significant maintenance or modifications, or as part of scheduled quality assurance testing.
Associated with equipment performance, OQ verifies that equipment, such as measuring devices, utilities, and manufacturing areas, performs according to specifications across operating ranges established by the manufacturer. The OQ process includes the following:
- Identification of critical operating parameters
- Description of experiments for critical variables
- Details about the sequence of the experiments
- Specification of necessary measuring equipment
- Acceptance criteria for the product
OQ testing can include:
- Activity triggers
- Alarms
- Automation features
- Calibration
- Computer and software systems
- Environmental conditions
- Flow
- Interlocks
- Keyboard controls
- Leveling and fluctuation
- Pressure
- Process controls
- Repeatability
- Safety
- Speed
- Timing
- Use of procedures by operating personnel
- Voltage and amperage levels
Performance Qualification (PQ)
PQ includes the verification and documentation of equipment’s performance. It verifies that all equipment is working within the accepted range and performs as expected in real-world conditions. PQ focuses on the manufacturing process, with the system testing done at operational capacity.
Faults that can be identified during PQ include:
- Excessive heat
- Excessive vibration or noise
- Pressure differentials
- Process media backflow
A robust PQ protocol ensures adherence to FDA and other regulations. Elements that should be included in a PQ protocol include:
- Acceptance criteria for PQ
- Analysis methodology that includes the justification for data and statistical tools to be used
- Calibration and validation tests that determine the consistency of quality throughout the production
- A data summary that articulates what needs to be analyzed or recorded while conducting testing, calibration, and validation
- Manufacturing conditions, including component inputs, operating parameters, equipment environment, and equipment limits
- Nonconformance contingency plans to address how to handle non-compliance and remediation procedures
- Operational procedures for personnel
- Performance measurement indicators and expected results
- Sampling plan that defines what methods should be used, during and in between the production batches
- Standard operating parameters and limits
- Test data to be gathered
- Test procedures for measuring equipment
- Time frame and schedule for gathering data
- Variability limits
Benefits of IQ, OQ, and PQ in FDA-Regulated Industries
- IQ—Reduces the risk that equipment was not installed correctly.
- OQ—Ensures that equipment operates according to specifications.
- PQ— Confirms that the workings, forces, and energy of the individual components of the equipment operate as one harmonious system.
Proven Framework for Quality Validation
IQ, OQ, and PQ validation procedures are required to prove that a product meets FDA and other regulatory standards. However, IQ, OQ, and PQ are beneficial to the manufacturing process for FDA-regulated industries.
The documents produced as part of these qualification processes are invaluable when an issue is identified. The qualification documents make it easier to retrace steps and identify the root cause of an issue.
While sometimes cumbersome, OQ, IQ, and PQ provide a proven validation framework that enables the quality and safety of medical devices.
Egnyte has experts ready to answer your questions. For more than a decade, Egnyte has helped more than 16,000 customers with millions of customers worldwide.
Last Updated: 5th December, 2021




