CDISC and CDISC Standards
What Is CDISC?
CDISC, or Clinical Data Interchange Standards Consortium, is a non-profit organization that develops data standards for clinical data collection, analysis, and exchange in collaboration with a wide range of experts from around the world. CDISC standards are free and used by researchers, pharmaceutical and biotech companies, agencies (e.g., FDA, PMDA, NMPA), and technology vendors. The standards are used to facilitate the accessibility, interoperability, and reusability of data to improve clinical research and global health.
What Are CDISC Standards?
CDISC standards focus on the core principles for defining clinical and non-clinical research data. Divided into four primary groups, the CDISC standards support data management through all phases of the research lifecycle.
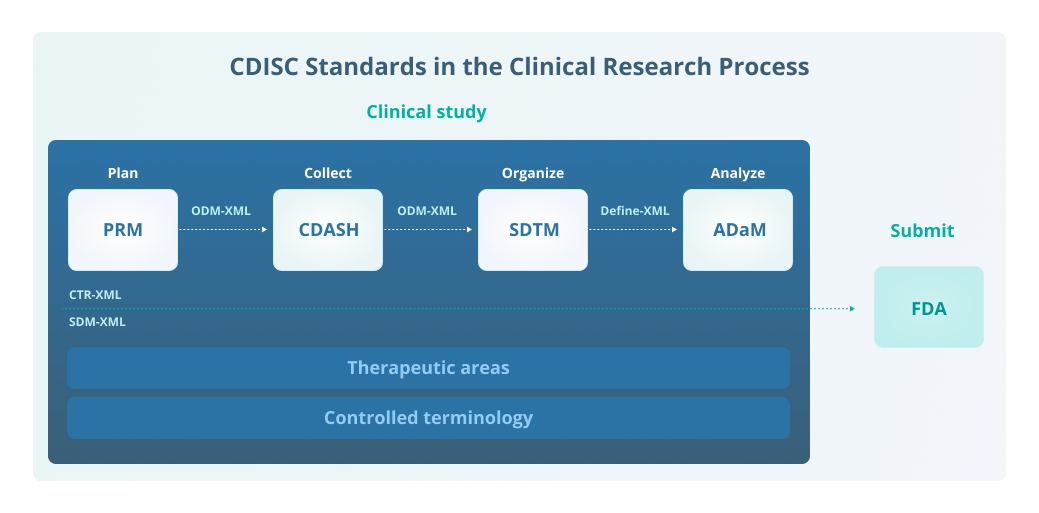
Four CDISC Standards Groups
- 1. CDISC Foundational or Content Standards
The CDISC foundational or content standards define what objects are allowed. These standards provide the basis for other CDISC standards that support data collection, management, analysis, and reporting.
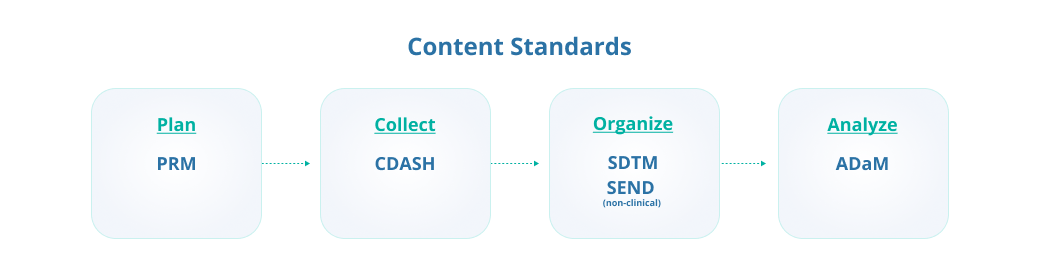
The Protocol Representation Model (PRM)
PRM organizes study protocols to meet study design, eligibility criteria, and requirements from registries, including ClinicalTrials.gov, World Health Organization (WHO), and EudraCT. It identifies and organizes items in a study protocol into a machine-readable structure.
Clinical Data Acquisition Standards Harmonization (CDASH)
CDASH provides guidance on the best structure to collect electronic case report form (eCRF) data consistently across studies and sponsors.
Study Data Tabulation Model (SDTM)
SDTM provides a standard for organizing study data in a common format to streamline the collection, management, analysis, and reporting by providing a data format standard to streamline data exchanges. SDTM consists of the core model and Implementation Guides (SDTM-IGs) that define how data from common domains should be represented (e.g., SDTMIG-MD for Medical Devices, SDTMIG-PGx for Pharmacogenomics and Pharmacogenetics).
Standard for Exchange of Non-Clinical Data (SEND)
SEND is the data standard for non-clinical studies that facilitates data exchanges (i.e., the non-clinical version of SDTM). It accommodates differences in clinical and non-clinical data.
The Analysis Dataset Model (ADaM)
The ADaM is used to standardize the presentation analysis data derived from SDTM datasets. ADaM defines dataset and metadata standards that support the efficient generation, replication, and review of clinical trial statistical analyses. It also supports traceability among analysis results, analysis data, and data represented in the SDTM.
- 2. Data Exchange Standards
The CDISC data exchange standards define how data is described to make it easier to share it.
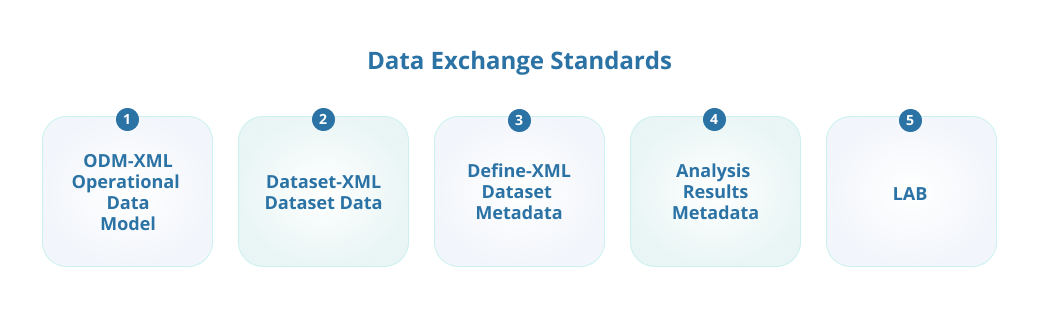
ODM-XML
Operational Data Model (ODM) is a vendor-neutral, platform-independent XML-based model used to exchange and archive study data, along with associated metadata, administrative data, reference data, and audit information. ODM-XML is used to represent study content, including data collected in eCRFs and electronic data capture (EDC) systems. Define-XML, Dataset-XML, and Analysis Results Metadata are implemented as extensions to ODM-XML.
Dataset-XML
Dataset-XML enables the communication of study datasets for regulatory submissions. It also facilitates other data interchange use cases, such as allowing a CRO to transmit CDISC-standard datasets (e.g., SDTM, ADaM, SEND), or any other type of tabular dataset to sponsor organizations.
Define-XML
Define-XML is a data model that standardizes descriptions of tabular dataset structures. Basically, define-XML describes the structure and content of data collected or submitted during the clinical trial process. It is used in conjunction with CDISC foundational or content standards.
Analysis Results Metadata (ARM)
ARM provides a means to trace results back to source material, such as key pieces used for analysis, datasets, data selection criteria, and even specific pages in an analysis plan.
Laboratory Data Model (LAB)
LAB standardization allows seamless data transfers between laboratories, CROs, and sponsor organizations.
- 3. Therapeutic Area Standards (TA)
Therapeutic area standards extend CDISC foundational or content standards to represent data for specific therapeutic areas (e.g., asthma, diabetes, multiple sclerosis). Therapeutic Area User Guides (TAUGs) are implementation guides for each therapeutic area that provide more detailed information.
- 4. Controlled Terminology (CT)
Controlled terminology is an evolving set of terminology standards published by CDISC in partnership with the National Cancer Institute (NCI) to ensure data consistency for PRM, CDASH, SDTM, SEND, and ADaM. It is a set of codelists and valid values that are used for items in datasets (e.g., if a question on the eCRF is to record sex, the allowed values are F (female), M (male), U (unknown).
Why is CDISC Important?
CDISC is important because it amplifies the impact and value of clinical data. It harmonizes data from diverse groups, making it interoperable across organizations and systems.
For existing studies, CDISC-compliant data can be pulled from multiple study sources and easily aggregated. In addition, future studies can easily access data from previous studies, thereby increasing the value and impact of clinical studies.
When Are CDISC Standards Required?
Many companies and organizations have adopted CDISC standards to take advantage of the many benefits or because partners or vendors require it. However, the more compelling cases when CDISC standards are required are when working with regulatory agencies. Currently, compliance with CDISC standards is required by the FDA, PMDA (Japan), and NMPA (China).
CDISC FAQ
What Does CDISC Do?
CDISC facilitates the exchange, acquisition, and archiving of data for biopharmaceutical product development. The requirements and guidelines released by CDISC influence the global standards for both non-clinical data and clinical data.
Who Belongs to CDISC and Why?
CDISC members are organizations seeking to maximize the impact of clinical research data. They use CDISC data standards to help make clinical data easier to understand and interpret. CDISC’s membership includes a variety of organizations associated with clinical research, including:
- Academic institutions
- Biotech firms
- Clinical research organizations
- Government agencies
- Healthcare providers
- Non-profit organizations
- Pharmaceutical companies
- Technology service providers
What are the benefits of implementing CDISC Standards?
Organizations that implement CDISC standards see improvements in:
- Cost savings
- Data quality
- Data sharing
- Innovation
- Operational efficiency
- Predictability
- Processes
- Traceability
Is CDISC required by the FDA?
Yes. The FDA requires studies to be submitted using “standardized data.” FDA has worked closely with CDISC to ensure that CDISC standards help regulatory reviewers to receive, process, review, and archive submissions more effectively. The FDA is a Platinum Member of CDISC Standards.
How are CDISC standards grouped?
CDISC standards can be grouped into four different areas:
- 1. Content standards
- 2. Data exchange standards
- 3. Therapeutic standards
- 4. Controlled terminology
What is the CDISC Library?
The CDISC library is a central repository where CDISC metadata standards are developed, integrated, and accessed. It provides CDISC content standards in a machine-readable format. By providing an API, the CDISC library facilitates the implementation of the standards with clinical trial software (e.g., a clinical trial management system or CTMS). Standards are accessible in a variety of formats, including RDF, XML, JSON, and CSV.
What is Controlled Terminology?
This provides direction about how to submit the collected data in the electronic dataset rather than what to collect. Controlled terminology provides a set of valid values from a CDISC-defined database. These values are what are required for submission to organizations that require CDISC compliance.
Egnyte has experts ready to answer your questions. For more than a decade, Egnyte has helped more than 16,000 customers with millions of customers worldwide.
Last Updated: 23rd November, 2021