
Egnyte for Life Sciences
More Science, Less Friction
Egnyte for Life Sciences gives biotechnology companies unified visibility and control into their most valuable asset — data.
A Unified Platform For Life Sciences Data and Documents
Egnyte for Life Sciences helps biotechnology companies connect R&D, business, and regulated data. Improve CRO collaboration, digitize Quality documentation, enhance workflows, and manage eTMFs while adhering to GxP standards and FDA 21 CFR Part 11 compliance.

The Most Innovative Companies Use Egnyte
Compliance + Collaboration
We needed a solution that would augment our workflows to keep the drug development on track, regardless of the location of each team. I had come across Egnyte a couple of times and with a little more investigation saw greater potential than performance alone.

Robert Kuhn
Director, IT Infrastructure & Security, Travere Therapeutics
No more data ‘hide and seek’
As a small molecule R&D organization, we have work going on internally and externally. As that data comes back in from the CRO, information is automatically stored in a logical folder structure so it is organized. There is no more hide and seek with our data.

Chris Moxham Ph.D.
Senior Vice President Discovery Research, Fulcrum Therapeutics
A centralized repository with compliance integrated
Bio-Techne needed a single source of truth for all files, everywhere. Security was important, not only for IP, but for data regulation compliance, including 21 CFR Part 11. The solution needed to be compliant, secure, scalable, user-friendly and cost-effective.

Aaron Froberg
Director of Global Infrastructure, Bio-Techne
Compliance, Collaboration, and Control
Meet Regulatory Compliance
Setting up a compliance envelope is easy to implement and manage with Egnyte. Audit trails track activity while GxP-compliant approval workflows enable digital document management and collaboration in a single interface.
Improve Collaboration
Supercharge collaboration on large files like SAS records, sequencing data, or DICOM imagery. Seamless integration with productivity and industry software streamlines collaboration on regulated content.
- GDPR
- CCPA
- New York Shield Act
- UK’s DPA
- Canada’s PIPEDA
- China's Personal Information Specification
- South Korea’s PIPA
- Australia’s PPA
Protect Valuable Information
Detect sensitive information like PII and PHI to comply with data privacy regulations like GDPR and CCPA and monitors for threats like ransomware. Get alerted to misclassified sensitive data so you can prevent data breaches and theft of intellectual property.
Approval & Signature Workflows
This capability provides a life sciences company with a single, compliant environment for designated stakeholders to collaborate on and, subsequently, approve regulated documents. Our flexible software provides a single environment for all functions - clinical, quality, regulatory, and manufacturing, to name a few. Further, automated notifications remind assignees of outstanding tasks and keep everyone up to date on progress.
Learn More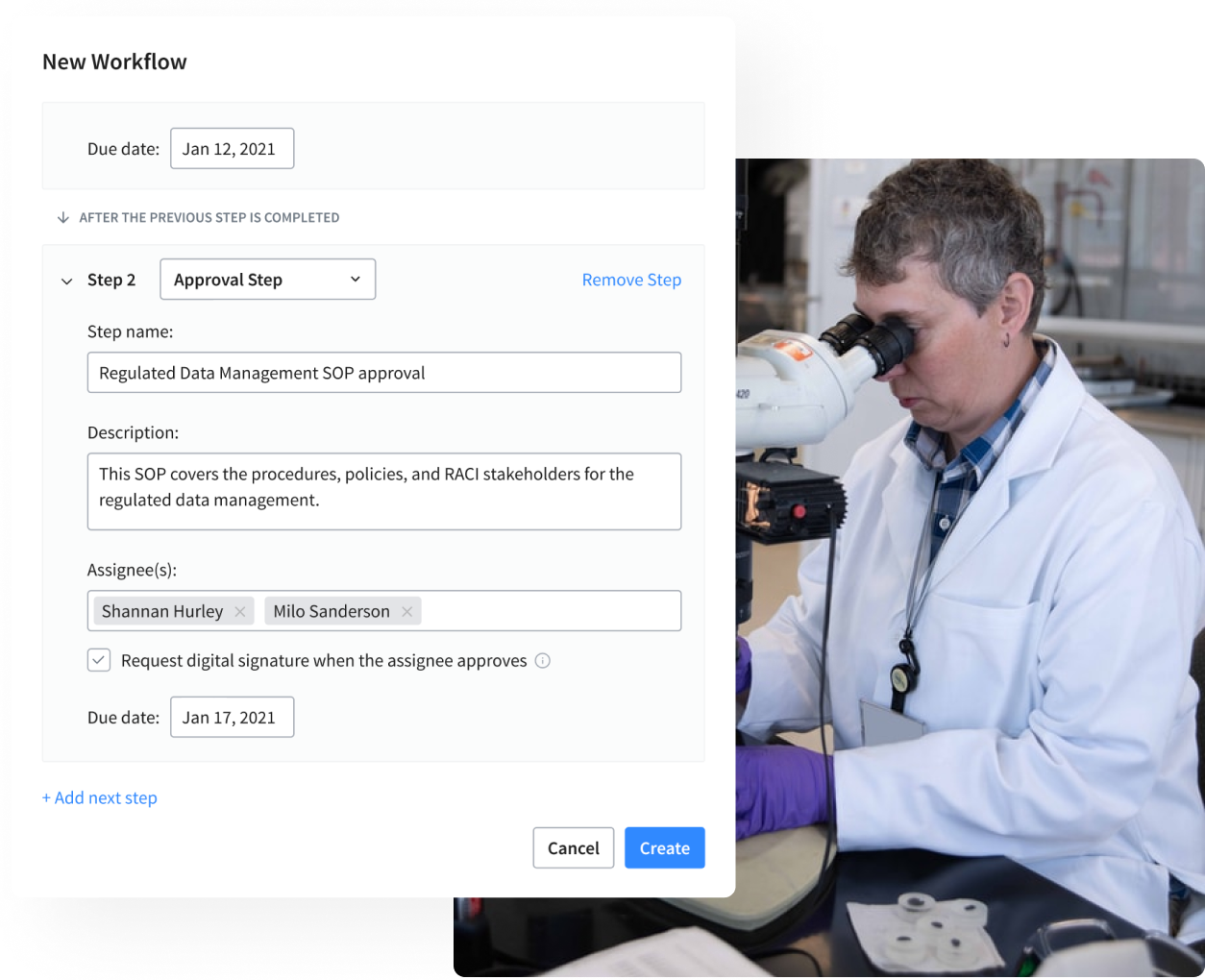
Egnyte Makes Things Easier for Life Sciences
Exchange Data with External Partners
Managing regulated data in one place reduces administrative overhead and improves security. Granular permissions allow partners limited access to the platform while allowing oversight of project progress.
Retrieve and Organize Trial Data
Maintaining one repository for trial content eliminates data “hide and seek” and facilitates organization of a TMF. Securely collect data from CROs and sites through designated folders or password protected links. Harmonize your program’s metadata through tags so that you can align with the DIA reference model.
Handle Large File Formats
It’s the best of both worlds, the speed of working with a local file and the universal access of cloud content. The hybrid capability of our life sciences platform ensures the fastest performance while at the office coupled with real-time updates and secure remote access.
GxP Audit Trails for FDA Compliance
Modern biotechs are moving compounds to the clinic at a faster pace than ever before. As a result, many companies struggle to ensure a strong compliance envelope as they transition from an R&D culture to a more-structured, regulated one. Learn more about how the Egnyte for Life Sciences platform is designed to meet Part 11 requirements, like audit trails, versioning, and reporting.
Watch NowEgnyte for Life Sciences Advisory Board
Along with our customers, the Egnyte for Life Sciences Advisory Board shapes the future direction of our product. It is composed of world-renowned scientists, biotech venture capitalists, drug developers, and a Nobel laureate.
Meet the teamPraised By Egnyte Users
on G2



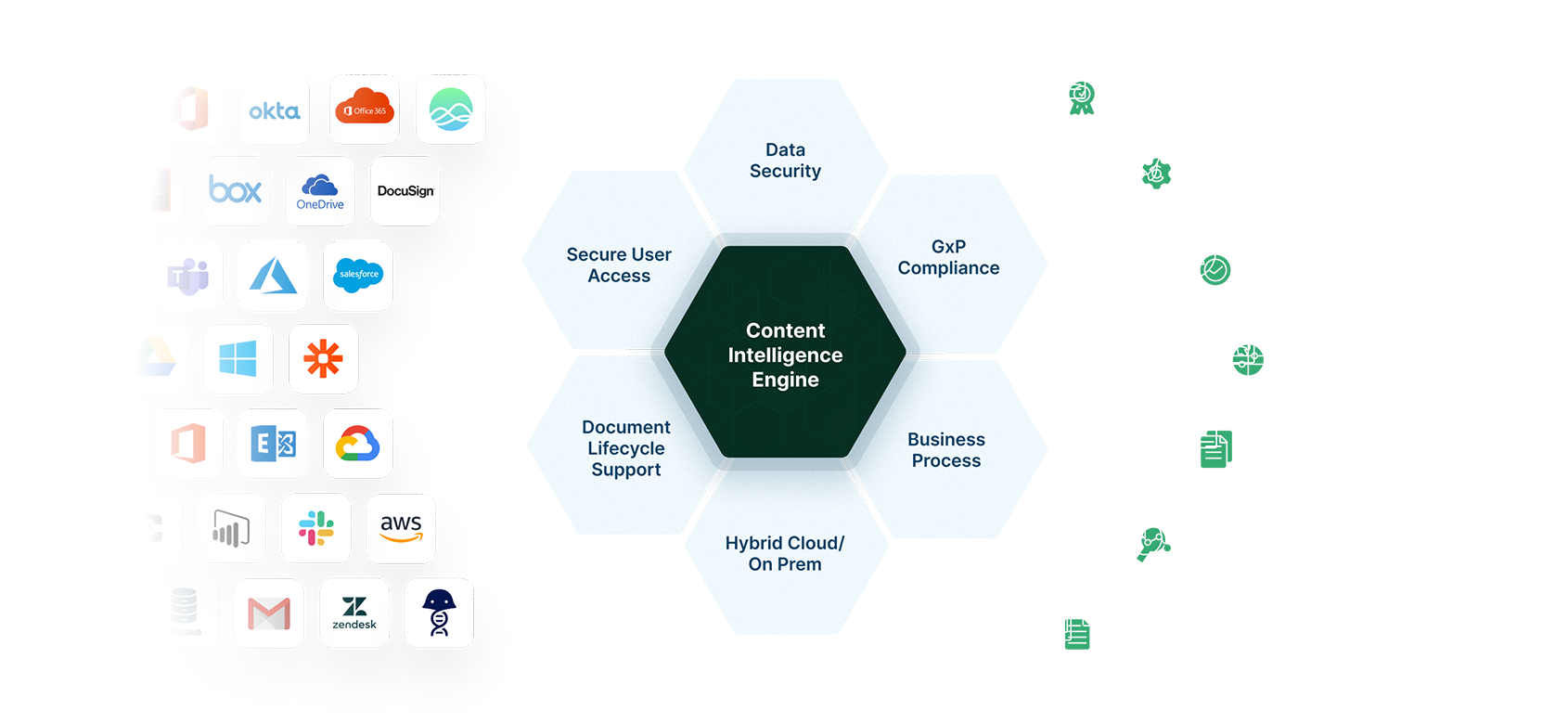
From a Single Platform, Egnyte for Life Sciences Enables

Additional Resources

ebook
GxP Buyer’s Guide
Read our buyer’s guide for a tangible framework to evaluate GxP solutions and ultimately, identify one or more that meet your specific needs.

White Paper
GxP Best Practices
Recent technological trends pose challenges for Quality & CSV teams. We’ve outlined best practices for tackling compliance issues when adopting new technologies.

eBook
Data Privacy Regulations Impacting the Life Sciences Industry
Data privacy laws around the world (CCPA, PIPA, APP) are harmonizing with GDPR’s principles-based approach. Are you ready?