
Use Egnyte to Manage Partner Agreement Reviews and Approvals
Co-development agreements are an important part of the negotiation process between companies that partner to develop, sell, or commercialize products and services. In life sciences, these contracts often contain sensitive data, including pharma alliance collaborations for drug treatment innovations, or healthcare software integrations to enable digital health interoperability.
Companies that have to manage reviews, approvals, and signatures throughout negotiations often find those efforts hampered by unsecure techniques used to share and access files. Traditional file sharing, often done via email, creates usability and data security issues. As a result, stakeholders often find themselves taking additional measures to:
- Constantly revisit the review progress, including tracking who has the latest version for review and which version is being reviewed
- Chase down signatures
- Manually scan and email counter-signatures
- Manage contract expiry upon development process completion or extension
- Protect confidential content, such as trade secrets or intellectual property
- Prevent users from accidentally sending sensitive content to unauthorized persons
With Egnyte, business development managers, alliance managers, and legal teams can confidently navigate the deal process with a unified platform for managing the lifecycle of partner agreement contracts. They get fast and secure access to collaboration tools to help produce co-development agreements, and when a deal is in place, Engyte ensures the agreement is accessible with the right viewing permissions and the right searchable location—allowing alliance managers to carry out or revisit the terms during the life of the partnership.
How To Manage Partner Agreements With Egnyte
Life sciences companies that rely on Egnyte have the advantage of using one platform to address many of the logistical challenges that come with negotiating co-development agreements.
Let’s take a closer look at how Egnyte acts as the hub for this process, and how easy it is to set policies and integrate with other tools so your business can protect sensitive data, accelerate reviews, and locate files from start to finish.
Protect Sensitive Information
During contract negotiation, there are various ways to use Egnyte to securely share documents outside your company.
If a company needs to share multiple files for capability review and due diligence, an admin can make project leads the owners of a project folder, which gives them full read/write/delete privileges for the folder content. Project leads can then manage folder permissions for any subfolders.

The administrator can also enforce two-factor authentication for external users, which enables more secure collaboration with partners.
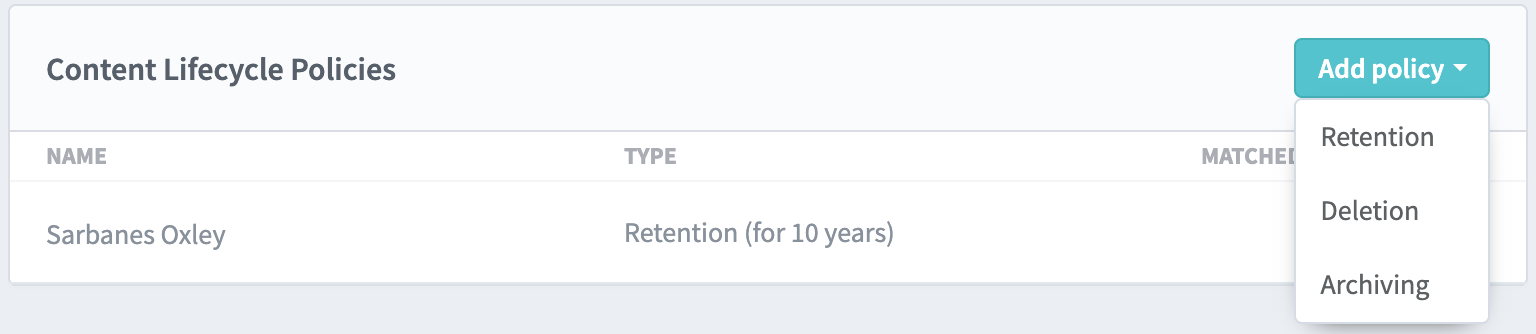
They can also set retention time frames and prevent deletion of folders or files by setting up content lifecycle policies. These policies allow the admin to select defined timelines for the retention, deletion or archival of content, and the process is then automatically enforced by the platform.
Review Contracts Faster
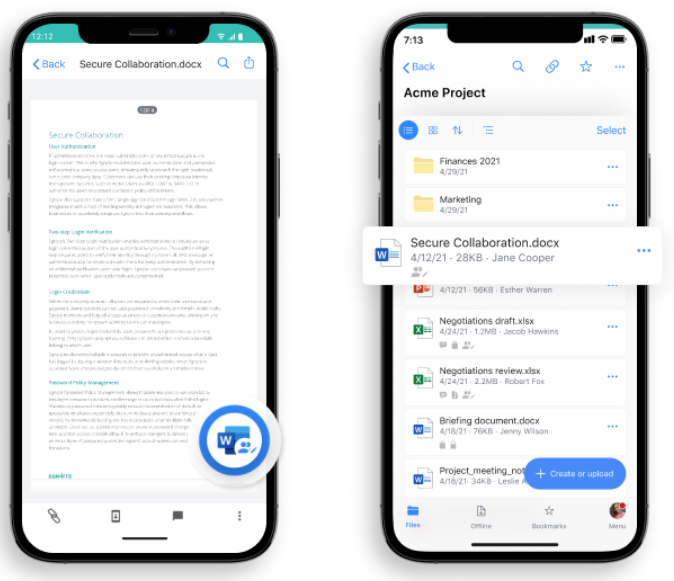
After reviewing due diligence documents and creating collaboration folders, partners can then easily collaborate on finalizing a co-development agreement. Simply assign the external collaborators as Egnyte Standard Users. This gives them sufficient access to collaborate on a folder’s files, but it blocks their visibility to your other content folders. Subsequently, project leaders can create a review and approval workflow for a contract document, including assigning users, and applying due dates for automatic notifications.

Project leaders can also collaborate by co-editing a contract directly with Microsoft 365 applications. To get started, they configure the Microsoft Office integration with Egnyte so access can be shared with external users. This process can also enable co-editing of Microsoft Office on Egnyte mobile devices.
Manage Electronic Signatures
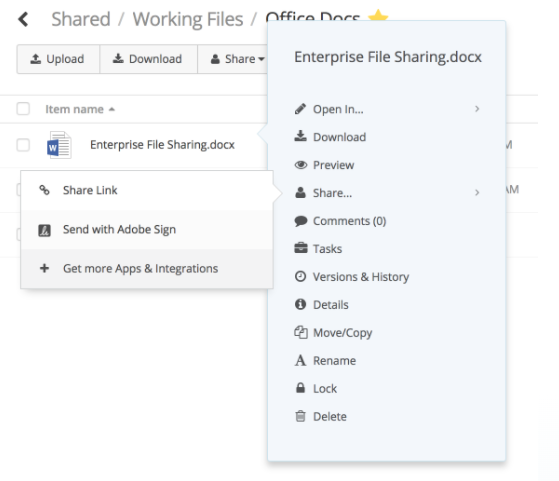
Once the review cycle is complete, the project leader can send a request for signatures through one of several Egnyte e-signature app integrations, including Adobe Sign.
To use Adobe Sign, first install Adobe Sign on your domain. Then, send documents from your Egnyte account to Adobe Sign.
When the external partner signs, a copy of the document will appear in your Egnyte account in the folder where the original document resides.
The e-signature integration is also accessible via mobile app, which saves time and reduces stress about getting deals signed before a deadline.
Easily Locate Your Contracts
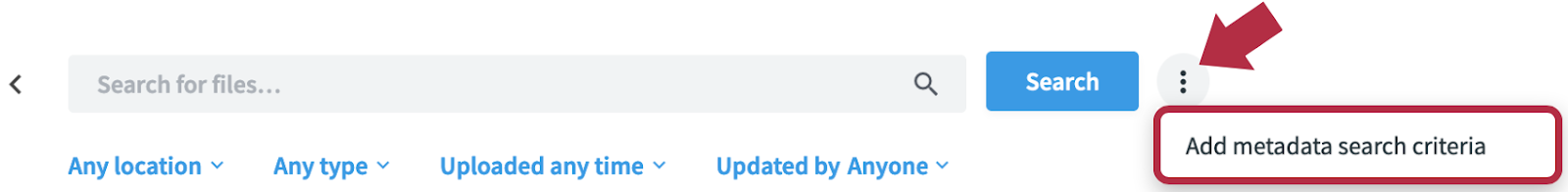
Egnyte makes it easy to search for files and folders. Project team members can enter a term or phrase, search by file type, or search by modified time.
And if you’re an admin, you can use metadata tagging to make it even easier to quickly identify and find the contract. Simply add metadata tagging once the document is finalized by annotating it with the date of the contract, who signed it, and the value of the deal. This ensures no obligations are forgotten, and once the metadata search criteria is set up, team members can search any metadata section and property you have added to a folder.
And that’s it. Using Egnyte, life sciences companies can secure and manage co-development agreements during the lifecycle of the partnership. For other options to manage the contract lifecycle, Egnyte also integrates with third-party applications for contract management, such as Ironclad, Formstack and DocuSign.






