Share Large Genomics Files With CROs To Support Clinical Programs
Increasingly, life science companies are applying omics-based testing to clinical trials. These tests support precision medicine models for the study of rare cancers and other diseases. Genomics research tests, for example, can help account for diverse drug responses and outcomes caused by genetic differences in trial participants. Bioinformatics and genomics research can also enable the development of improved diagnostics and more effective drug therapeutic strategies to help identify the right patients for the drug.
These techniques show great promise for drug research, but they create logistical challenges when pharmaceutical companies contract with clinical research organizations (CROs) to perform the tests. The clinical data manager must collect the data from CROs, then validate its integrity and accuracy—all of which typically requires high-performance computing for the rapid transfer and analysis of gigabytes of data. This creates a need for secure data workflows and large file storage to make results available faster.
Egnyte helps clinical data managers, who are integral to the success of clinical trials implementations, by providing data transfer and file access tools to collaborate with CROs and process large data sets.
Manage Large File Transfers From CROs With Egnyte
As a clinical data manager, you can enable CROs to transfer large analytical datasets to your company using Egnyte, in one of two ways:
- Send them a simple upload request link for collaboration, removing the need to create an additional user account
- Assign the external partner a limited, role-based permission with access to designated folders
This seamless upload and transfer process to cloud-based repositories in Egnyte gives you fast access to large data sets for scientific analysis. Egnyte facilitates file sharing with encryption-based security, so you know your data is secure in transit and at rest.
Let’s take a closer look at how you can administer your Egnyte domain to support these types of secure transfers.
Request File Uploads Without Creating New User Profiles
If the CRO only needs to upload a few files, there is an easy and secure way to request files using Egnyte. Simply go to the designated folder in Egnyte and click on Request Files from Others in the Share dropdown menu.
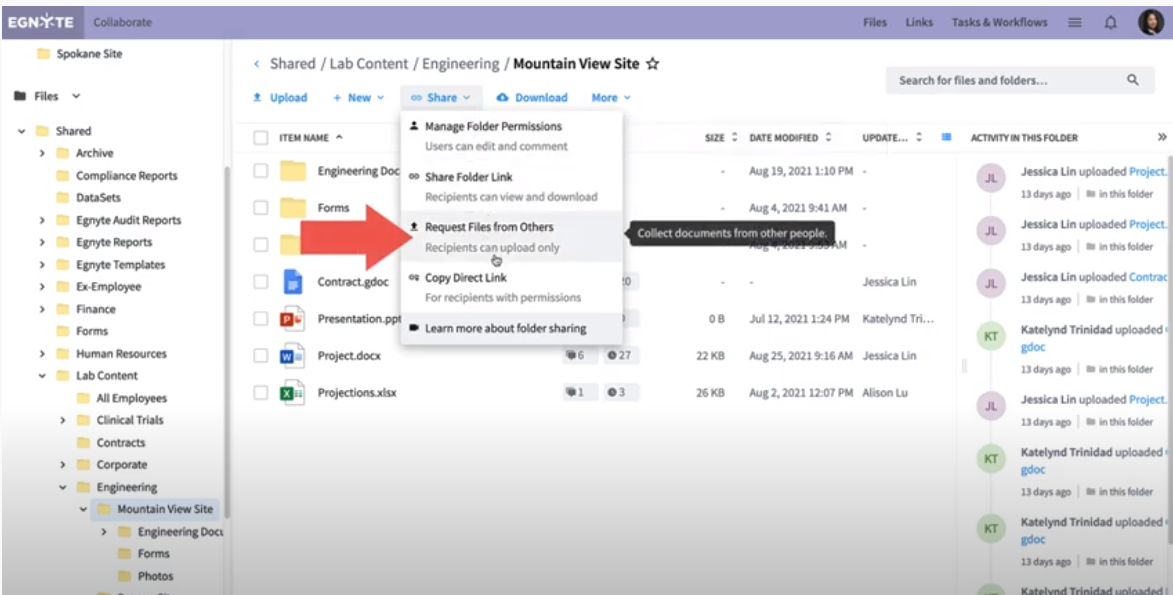
This will generate an upload link, which you can use to easily collaborate with an external partner, without having to go through the hassle of creating a user profile for them.
This secure link eliminates the need for email attachments, which have file size limitations, and FTP sites, which require designated servers and more administrative work. You can also assign automatic expiry to the upload link to ensure it becomes inactive after a set amount of time. And because you don’t need to create a user profile, you don’t have to worry about the partner accessing anything else within your system.
Assign Role-based Permissions for CROs
If you collaborate frequently with a given CRO, you can also assign them as a user with limited access to files, by assigning them as a Standard User.
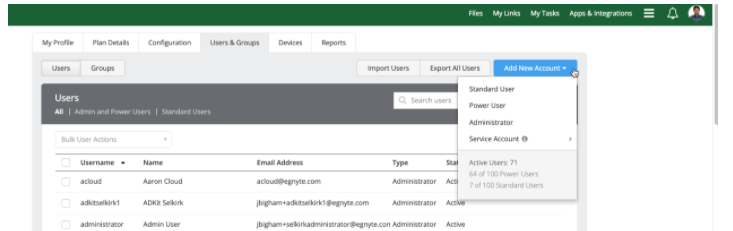
You can assign Standard User permission in one of two ways. You can go to Users & Groups and click on Add New Account and fill out the necessary fields. You can also create a Standard User directly from your folder access. Click on the Share dropdown menu, select Manage Folder Permissions, and enter the fields. The CRO will receive an email notification so they can start collaborating.
When you assign the CRO as a Standard User they will be able to collaborate with Egnyte users to share, view, and comment on files. However, to keep your data more secure, Standard Users don’t have the same permissions as internal users. For example, they are not given their own private folder, nor can they share file or folder links.
The assigned role-based permission also allows your IT administrators to track the behavior of files and individuals. Administrators can also use reporting tools to provide a GxP audit trail.
At project conclusion, when those files or folders are no longer needed for collaboration, IT can easily disable a user permission in the Egnyte folder. Alternatively, if you know your project conclusion dates, you can also assign expiration dates to the user profile.
Egnyte makes large file transfers quick, secure, and simple, and it’s especially attractive for those looking to retire old clunky sFTP servers. It also enables you to preview large files without having to pull them from the cloud. Of course, if you need to use FTP for file transfer, Egnyte is compatible with FTP clients, too.
Speed Up Collaboration
Once the CRO uploads the data to the Egnyte cloud, it is available to anyone with access. Additionally, you can use Egnyte to cache the file locally, either on your computer or on a local server, so that you and your colleagues can access it quickly.
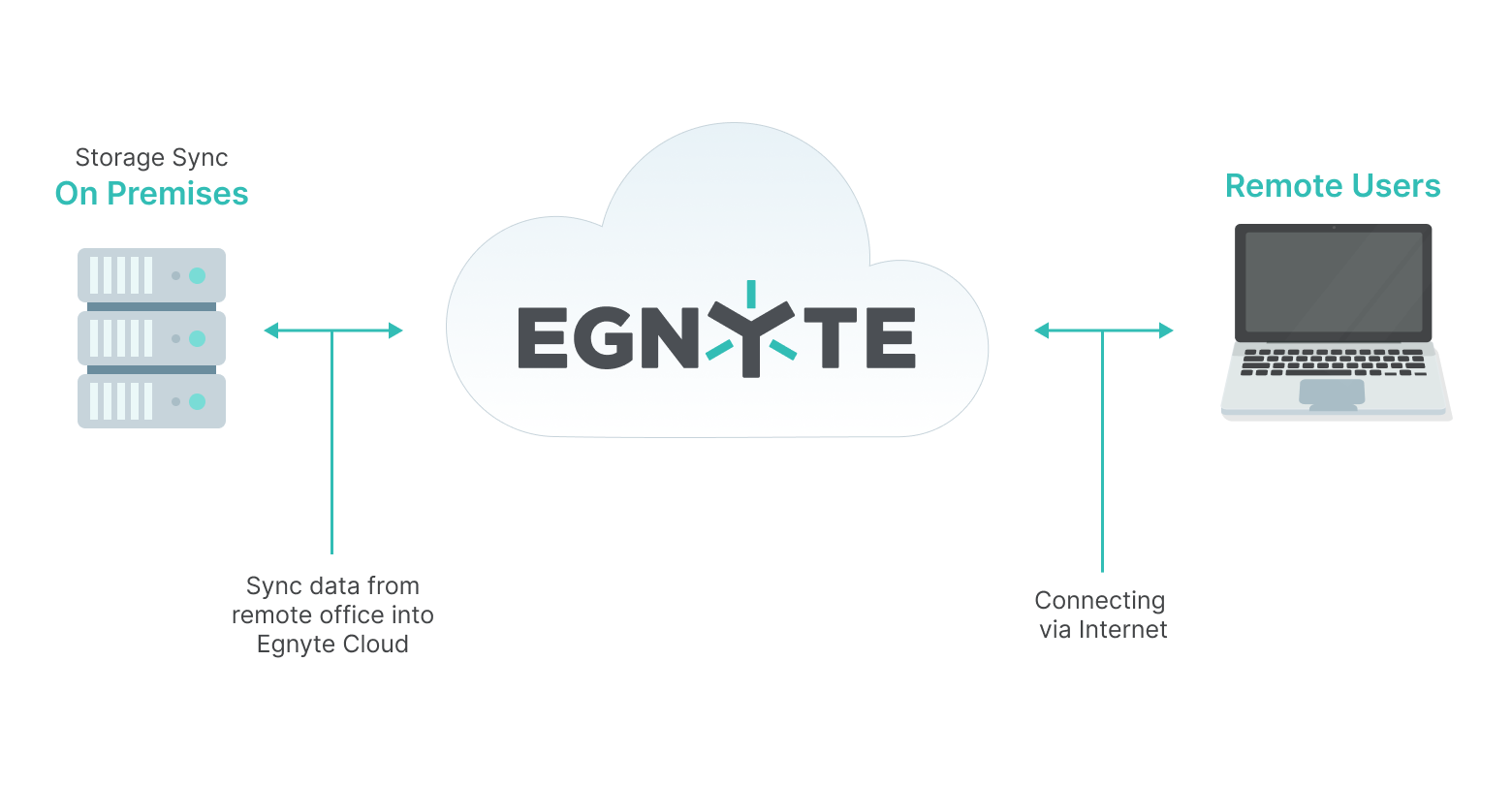
Here’s how it works: when data is uploaded to a folder in the Egnyte cloud, if there are other on-prem devices that are synchronized to that folder, it will pull down that file immediately. The real-time synchronization gives you and your colleagues the latest data, as well as GxP-compliant audit trail of data access. It also gives you the comfort of knowing you are all working on the most updated files, whether you work at the office or remotely, for a single source of truth.
You now have all the benefits of a cloud-hosted content—including remote accessibility, real-time updates, and audit trails—without the drawback of delays caused by slow data transfers. As a result, you’re able to seamlessly collaborate with CROs that need to upload multi-gigabyte files such as genomics data.