Improve Clinical Trial Monitoring with Egnyte
Clinical trial monitors are vital to the efficient collection of high-quality data. They deploy trial information and protocol amendments from the sponsor to the sites, which ensures study progress. They also support endpoint analysis by collecting source data and by verifying adherence to the trial protocol.
But the sheer volume and velocity of data being created by modern clinical trials create significant challenges for life sciences firms—a problem that hits clinical research associates (CRAs) harder than anyone else.
It’s no wonder why CRAs eventually wear down. They’re responsible for reviewing each of the hundreds of thousands of source documents created during a clinical trial, and those documents are often poor facsimiles of originals, with illegible writing and confusing changes.
How Egnyte Helps CRAs
With burnout such a problem and experienced staff turnover a huge drag on your trial, it is important to find ways to help your monitors maintain a healthy work-life balance while making their work more fulfilling. Egnyte helps CRAs streamline the review of source data and improves the ability to deploy protocol changes to sites. This will improve the overall data quality, safety, and operation of your trial.
Egnyte’s AI engine offers life-science-specific algorithms as well as custom patterns for study-specific applications that offload some of the monitor’s more tedious tasks. From sensitive data detection—like PHI or PII inside trial data—to telling the difference between a completed document and a placeholder document within a TMF, Egnyte can review source data as it is submitted and flag out-of-range or non-compliant documents for further review.
As a result, CRAs can concentrate on higher-value risk-based tasks, act more quickly to address adverse events and safety issues, and focus on value-based work activities that increase job fulfillment and decrease burnout. When coupled with risk-based monitoring, Egnyte for Life Sciences adds an extra level of vigilance.
Here are just a few examples to illustrate how Egnyte can help.
Quality Documentation Management
Sharing SOPs with dozens of sites through email can be tedious and lead to poor version control. Instead, centralize the documents in Egnyte’s Quality portal, so sites always have access to the latest document and can see upcoming effective documents.
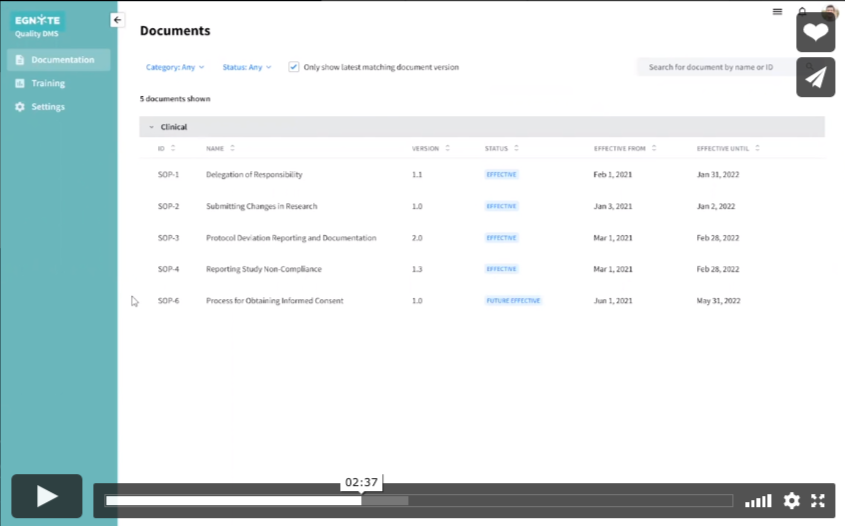
Keeping sites up to date is a straightforward process.
- All effective or future-effective documents are listed on the “Documentation” section
- All documents the user has access to are categorized and listed with their effective date and when they will be retired
- Clicking on any of the documents will open up a preview
- The preview will include its status and effective date in the PDF and a 21 CFR Part 11-compliant signature page
New effective documents can also trigger notifications so every site is up to date with protocol amendments.
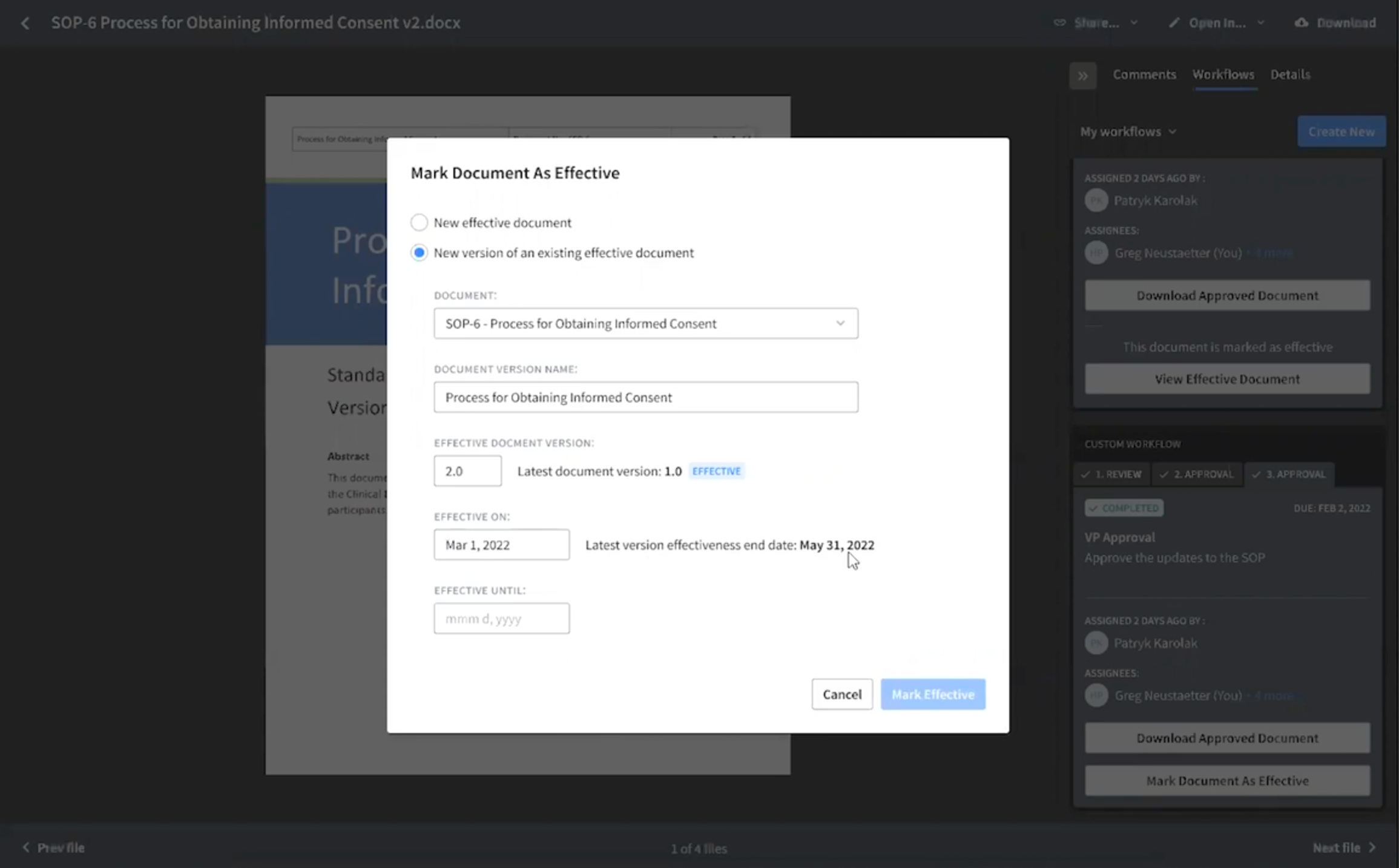
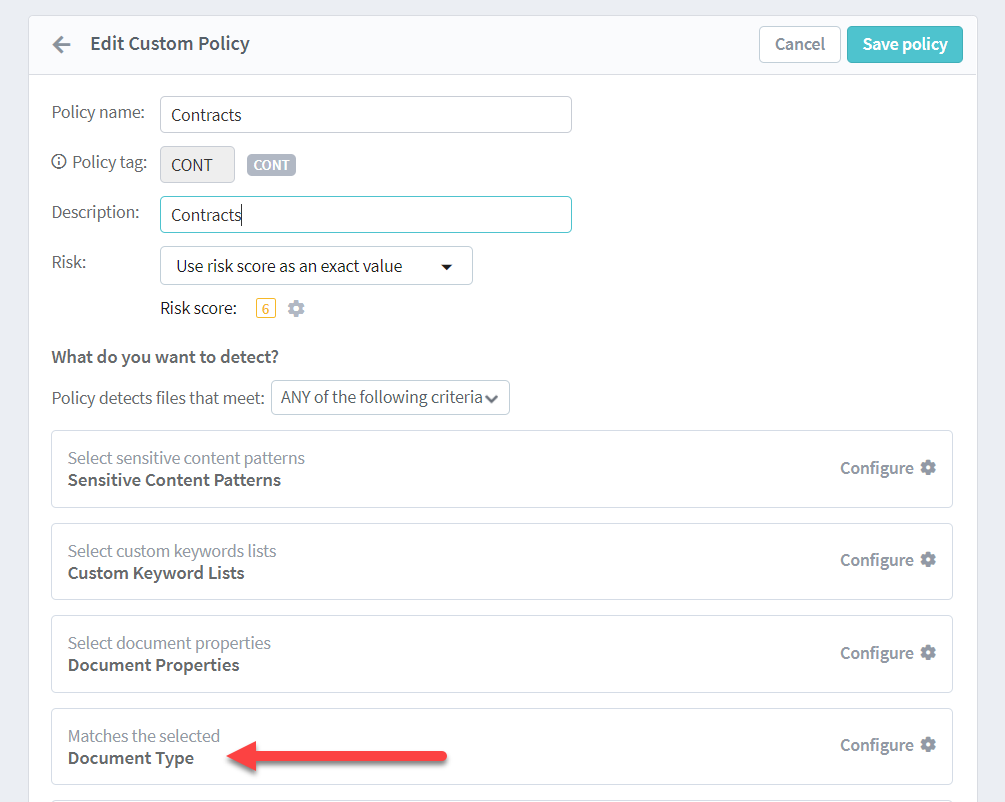
Uploading new quality documents is as easy. Just follow these steps:
- Select the category and subcategory that the document lives in. Categories make it easy to assign who gets to see which documents.
- Select the document you want to update. If it’s new, select the “New Document” radio button instead.
- Choose when you want the document to become effective—today or at some future date.
- Select the file you want to become effective and hit Submit.
By maintaining a central location for effective documents, sites will spend less time finding the documents they need to operate. This also reduces your administrative burden of knowing who has signed off on the new documents.
Sensitive Data Detection
Sites must keep trial data free of PII/PHI to ensure the privacy of trial participants and maintain trust. However, reviewing hundreds of thousands of documents for personal information is a tedious task. Egnyte streamlines the process by scanning and flagging possible privacy violations for manual review.
This additional level of privacy protection is extremely useful, especially for risk-based monitoring applications. With Egnyte, you can use pre-built filters, including detection for PII like emails and phone numbers, PHI like health record numbers, or insurance billing information. You can also create custom filters for data that’s unique to your study.

Here is how it works:
Open the Sensitive Content tab to view the file name, policies matched, and the risk level for each of the files found. Select the folder location and click Show detected content from the details pane.
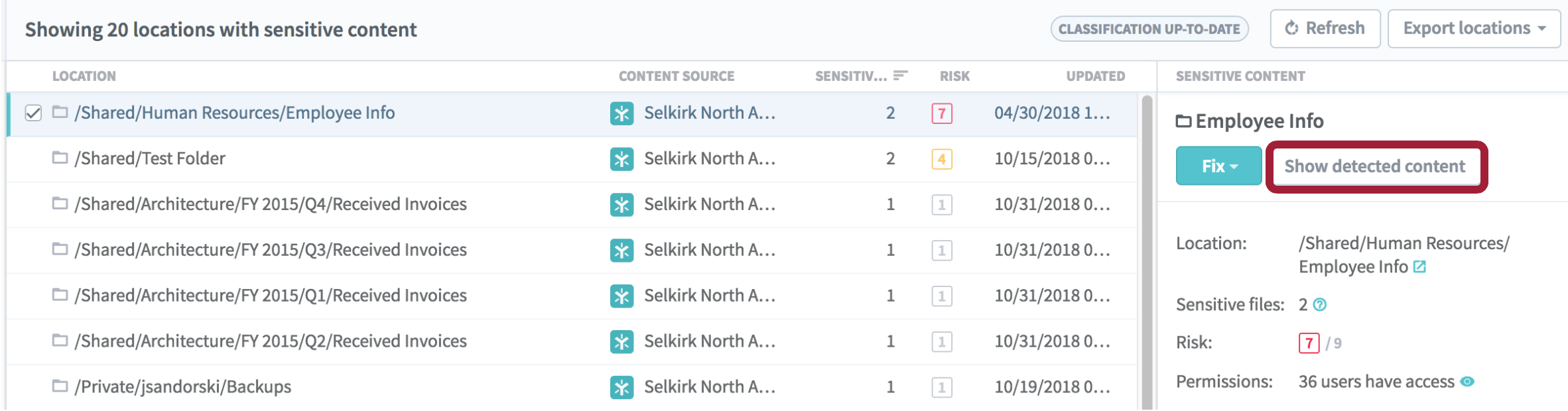
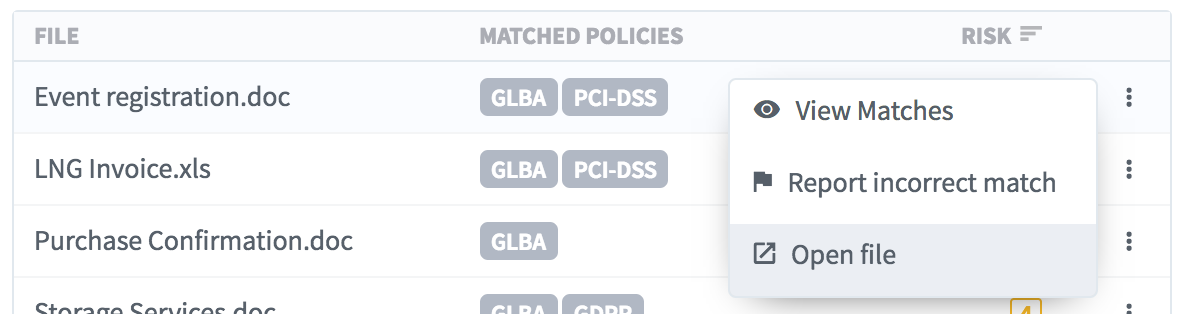
From this report, you can click Open folder or click Export to see the list of files as a CSV. For each file in the list, you can take any of the following actions from the drop-down at the right end of its row:
- Open the sensitive file in a new browser tab.
- Reporting a false positive: If the file is not truly sensitive, you can provide this feedback to Egnyte by reporting it as an incorrect match.
- Dive deeper into each file using the Sensitive File Viewer to view the actual sensitive content matches by either clicking on the file’s row or from the drop-down.
Clean Up and Classify Documents
Illegible or misplaced documents are the bane of any monitor’s existence. Whether it’s scanned improperly, has multiple changes, or just has poor handwriting, it is important to determine what is actually recorded in the source document in order to compare it to what is stored in the trial record. If the document is placed in the wrong folder or misread, it can often go unnoticed and overlooked.
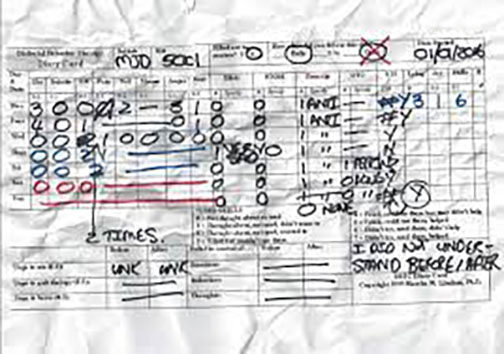
Egnyte can automatically rotate, adjust brightness, and apply optical character recognition to hard-to-read documents so they’re more legible and indexed for easy searching. Unfortunately, really bad handwriting still has to be deciphered with help of the data collector.
At the same time images are scanned and enhanced, Egnyte’s Content Intelligence Engine uses machine learning and your pre-defined triggers to assess those documents and ensure they’re stored in the right place. Egnyte will also flag documents that are improperly classified for manual review, in order to make sure they are stored correctly.
To enable Content Classification:
- Turn on Content Classification in your Settings page. You can have select folders scanned or the whole domain.
- Egnyte will automatically scan PDFs, JPGs, and more to evaluate legibility.
- If the document is illegible, Egnyte’s algorithms will attempt to make it easier to read.
- Egnyte will index the contents in order to make it searchable.
- The document will be compared to others like it to determine classification.
- Sensitive content will be surfaced if it is found.