Egnyte’s Life Sciences Summit Coming this September
With so much activity around improving our current Egnyte for Life Sciences product lineup and working hard to develop new products, we thought it was a good time to share how Egnyte is helping life science organizations adapt to new data management challenges.
We are hosting Egnyte for Life Sciences Summit, a one-day virtual event to be held Tuesday, Sept. 14. Sessions will include industry trends from IDC analysts, upcoming product innovations, and ways to navigate the practical application of data management and cybersecurity techniques for life sciences companies.
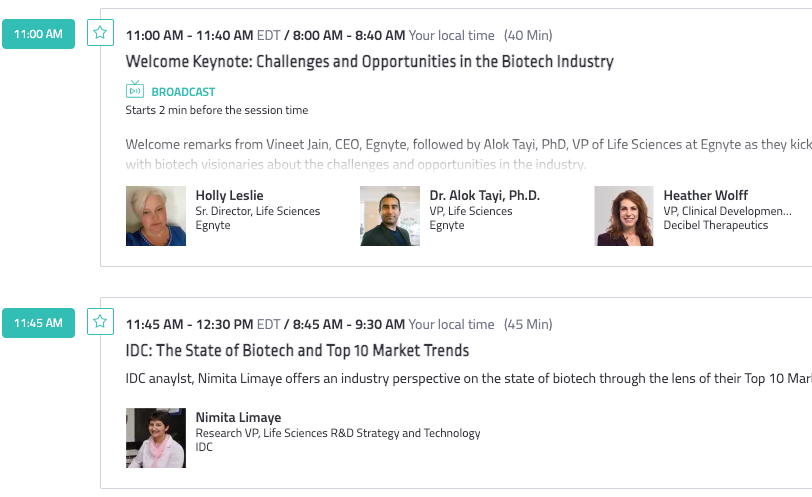
Register: Egnyte for Life Sciences Summit
The summit will feature a look at the MIT Tech Review’s Predictions for AI-led Drug Development, with guests from Calithera and IQVIA discussing how AI is transforming the way clinical trials work.
Life science visionaries, market analysts, Egnyte leadership team members and peers in the life sciences space will also be featured at the event, giving ample opportunity for customers to engage with like-minded individuals on how to enhance the future of biotechnology.
"In a year when life sciences made front page news, and investment in new and emerging life sciences firms reached record levels, we felt it was high time to focus a significant amount of our own energy and innovation on helping these firms achieve their missions even faster,” said Vineet Jain, Co-Founder and Chief Executive Officer at Egnyte. “We are inspired every day by our hundreds of life sciences customers and are excited to introduce at Summit several new products that will enable them to maintain data quality and integrity at the highest level.”
Egnyte’s multi-cloud content platform gives life science companies the tools to manage content-rich workflows for clinical studies, quality documentation, regulatory submissions and more. Working in a highly regulated industry, life science professionals will find value in Egnyte’s offering to provide quality documentation and regulated data that can meet GxP, FDA and EMA requirements.
Registration is free for those in the biotech, medical device, diagnostic, or related service organizations. Learn more at the Egnyte for Life Sciences Summit registration page.