Egnyte for Life Sciences: Compliance, Collaboration, and Governance
Today marks the release of Egnyte for Life Sciences, a unified data collaboration platform to serve those advancing the science of health. In recent years, Egnyte’s team of industry veterans has listened closely to companies, customizing solutions to ensure regulatory compliance, improve collaboration, and provide more control over your company’s most valuable asset: data.
Regulatory Compliance
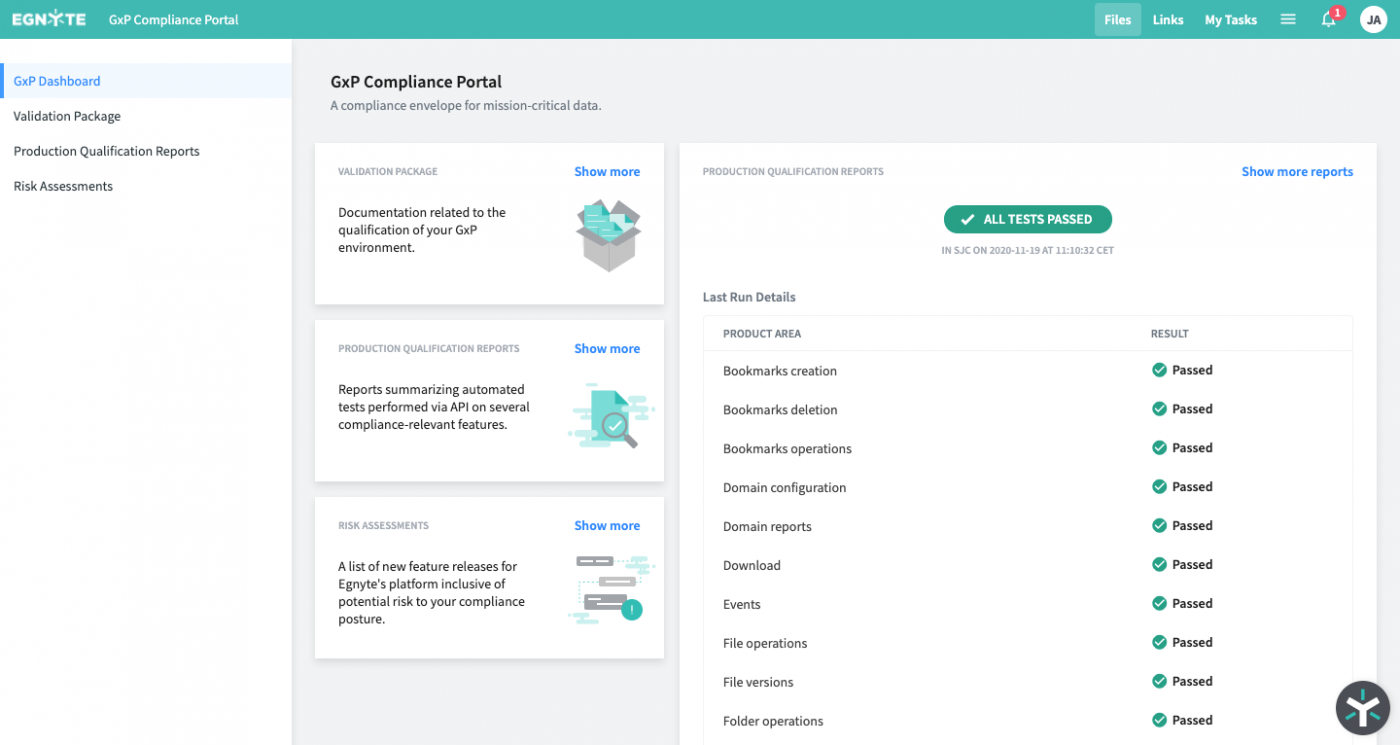
A major component of Egnyte for Life Science is support for GxP-compliance. Egnyte’s platform conforms to FDA 21 CFR Part 11 guidance and the EU’s Annex 11 directive that define how records and signatures are handled within electronic applications. The flexibility of Egnyte means you can comply with regulatory requirements, like audit trails, without distracting from your current workflows. Along with our audit trail functionally, you can create GxP-compliant documents through a review and approval process that amends electronic signatures to documents each time they are changed and approved.
Egnyte supports industry-standard workflows and data structures to streamline data collection and storage, as well as separate repositories for regulated and unregulated data. Whether you are working from the road, in the lab, or at a site, the files live on our compliant servers, so there is no need to worry about version control or overwritten data.
“There has been an explosion of data types, and the data sets we work with are quite large, things like high-content imaging and sequence data,” said Chris Moxham, Ph.D., Senior Vice President of Discovery at Fulcrum Therapeutics, a clinical-stage biopharmaceutical company. “The ability for Egnyte to store the data files on a compliant platform and provide seamless access to scientists so we can create knowledge and move forward has been invaluable.”
Secure Collaboration
From joint research to distributed clinical trial sites, virtual labs to remote monitoring, the accessibility of massive data sets among clinical partners kept the industry moving forward and sped up the development of treatments. This is a trend that predates the current COVID restrictions. But as the number of partners per project continued to increase year after year, so did the need to share data with an increasing number of parties like CROs and CMOs. On top of the increasing number of partners, the amount and diversity of data has increased. Large files and collections of files like DICOM formats are increasingly common. Instrumentation data and labs need to be analyzed promptly. This essential need to share massive data sets opens companies up to vulnerabilities and lapses in compliance.
Egnyte gives you the control to mitigate this risk. Features like granular permissions, unidirectional data flow, and expiring links mean that you have control over who has access to your data and what they can do with it. Robust reporting and alerts give you real-time visibility into suspicious activity and misclassified documents. Egnyte conforms to your organization’s data policy without giving up access or collaboration.
“As a small molecule R&D organization, we have work going on in biology and medicinal chemistry internally,” said Moxham, “but from a synthesis perspective, that work is going on externally. As that data comes back in from the CRO, information is automatically stored in a logical folder structure so it is organized. There is no more hide and seek with our data.”
Data Governance
2020 has been a transformative and challenging year for data security in the Life Sciences industry. An unprecedented number of malware, ransomware, and phishing attacks have hit healthcare companies. The results have been devastating. Research has ground to a halt. Clinical trials, including COVID trials, have been paused. Some of the data that supported such promising medicines is lost forever. Many organizations, either affected or identified that this is an unacceptable risk, have chosen Egnyte to help them protect their data from cyberattacks like this. This is yet another complexity for IT teams as they are gaining clarity about what GDPR and CCPA mean for their data governance protocols, and the penalties for non-compliance.

Egnyte helps companies understand who has access to their data and what they are doing with it. The ability to identify vulnerabilities and proactively plug holes reduces risk while increasing productivity. Integration into common productivity software means that all your data is protected without distracting you from your work. From common SaaS-based applications like Microsoft, Salesforce, and Google, to industry-specific tools like Benchling, it is easy to gain control and get comprehensive
visibility into how your data is being used and who
has access.
Why Egnyte for Life Sciences Matters
Life Science companies have always been a core part of Egnyte’s 16,000-strong customer base. The Egnyte platform is fundamental in the operations of over 400 biotechs and pharma companies, medical device manufacturers, and technology and service providers in the healthcare space. Egnyte customers are pushing the frontiers of medicine across cardiovascular disease, oncology, rare diseases, and vaccines—including COVID-19—across the globe.
Looking to the future, Egnyte will continue to build expertise and tailored products for Life Sciences customers with the guidance of a new Life Sciences Advisory Board. It is composed of preclinical scientists, practitioners of data management, thought leaders within clinical operations, a Nobel Laureate, and visionaries from the industry, who will guide Egnyte’s future product direction.
The need for a collaboration network that is overlaid with compliance and governance features has become exponentially more critical for life sciences organizations. Egnyte is committed to meeting these requirements and providing distributed workforces with the ease of use and security that is paramount to continued innovation.
For more information about how Egnyte can help further your research, visit Egnyte for Life Sciences.