Manage Clinical Trial Site Submissions with Egnyte
The volume, variety, and velocity of data being collected in clinical trials is constantly increasing. It regularly surpasses what any one person or even a team of people can process, organize and monitor.
Companies can no longer throw people at the problem, which is why many have turned to automation and AI to fill the gap. In fact, 92% of biotechs report that the pace of big data/AI investment in their organizations is accelerating, according to the New Vantage Partners’ 2021 Executive Survey—up 40% from the 2020 survey.
Many biotechs have turned to Egnyte to address the data deluge problem and reduce their administrative burden. As a result, they've made it easier for sites to submit their source data, which lets everyone focus on the high-value work that really moves projects toward study close.
How To Manage Site Submission With Egnyte
Clinical trial teams typically work with dozens of sites to ensure enough data is collected to support one or more endpoints. However, these teams lack the internal resources to coordinate, ingest, process, and track sites' workflows and all the data they produce.
With Egnyte, you can automate much of that process and leverage a variety of integrations to further streamline site submissions. This reduces the number of resources needed to track, classify, and organize data dumps into an eTMF. Here is how it works.
Customize Access for Sites and Sponsors
Each site is set up with a folder, which is only accessible to that site and the sponsor. The sponsor can see all site data, giving them a holistic view of all the trial data in one central repository. Conversely, each site can only see the files it has submitted, eliminating the possibility of overwriting other sites’ data or misclassification.
Because sites can only access their files, any potential damage from a cyberattack or insider threat is localized to that folder and not to the whole trial. This setup also improves tracking. It is much easier to see which milestones each site has hit and what is remaining, in order to gauge trial progress.
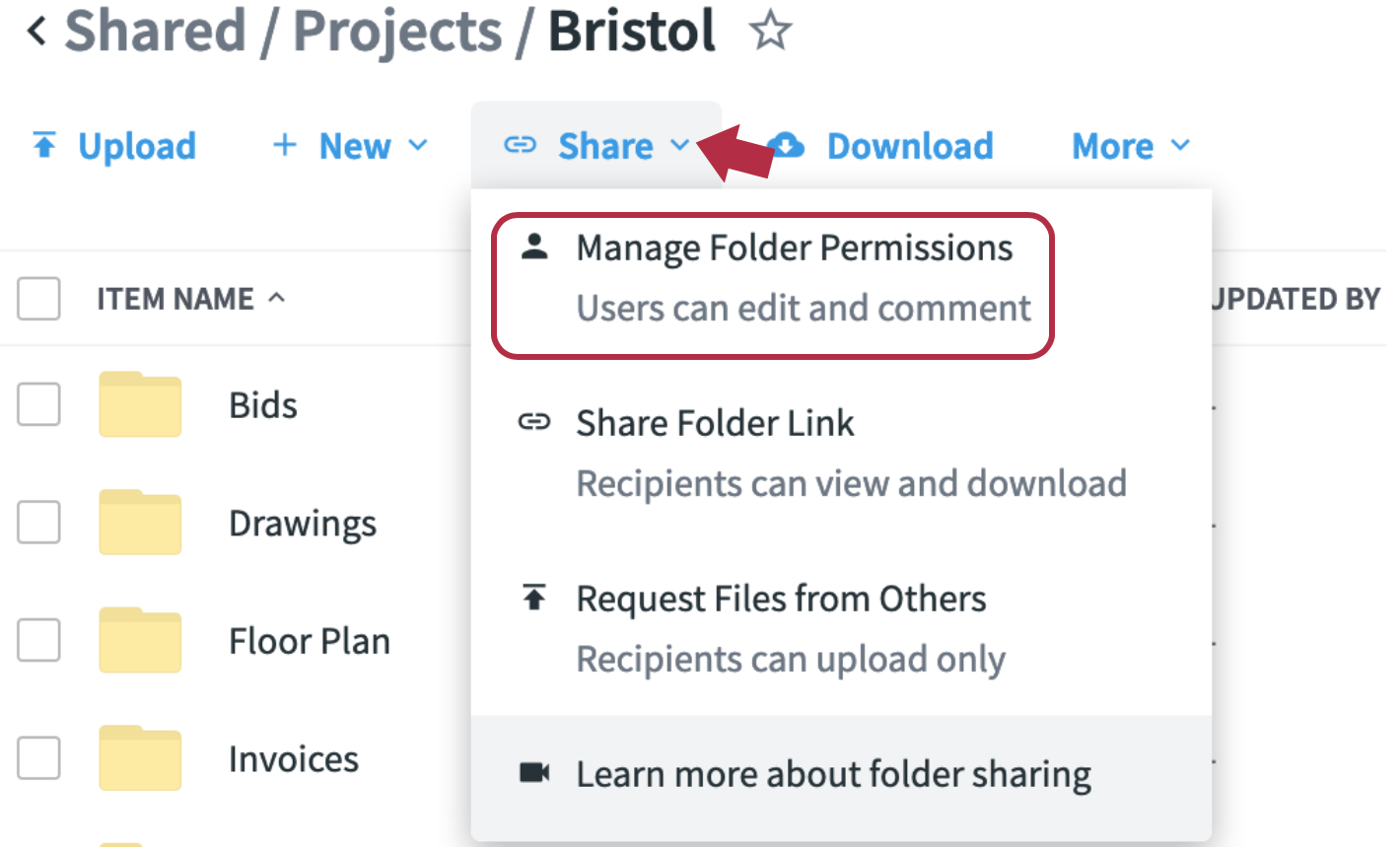
You can create the folder and set permissions through a few quick steps:
- In your project folder, expand the + New dropdown and select Folder
- Name the new folder after the site description, and press Create
- Hover over your new folder and click on the link icon, then on manage Folder Permissions
- Add the email addresses of site staff you want to be able to upload data
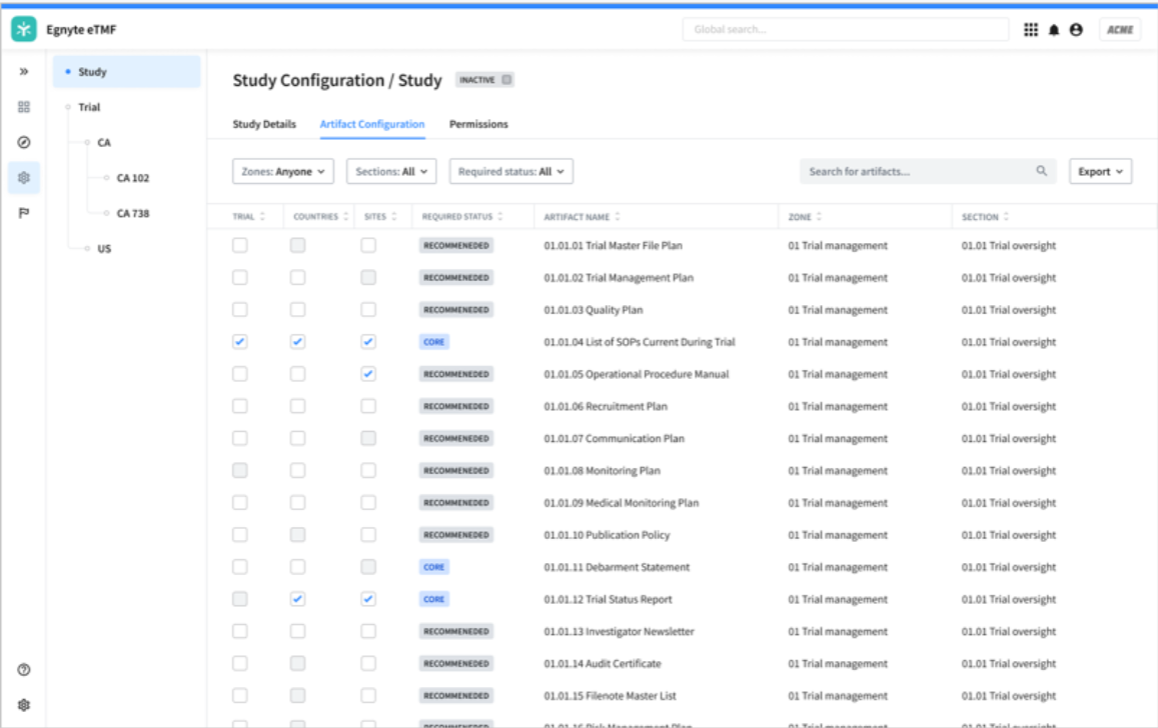
Tailor Reference Models and Trial Artifacts
You can also define the artifacts that will be uploaded, which makes classification quicker and more automated. Whether you choose a stock DIA model or one customized for your own specifications, you’ll have out-of-the-box templates to kick start your project. At this stage, you can:
- Use the DIA reference model as a template and select from 249 standard artifacts, from CRFs to specimen labels
- Add customized artifacts to accommodate your study design
- Apply submission rules to specify which are relevant at the trial, country, or site level
After site teams upload documents, Egnyte uses machine learning to automatically classify those documents based on available artifacts and rules. This removes the laborious process of manually classifying files, making it easier to assemble the eTMF, track trial milestones, and respond to compliance audits.
Increase Flexibility for Existing Site Workflows
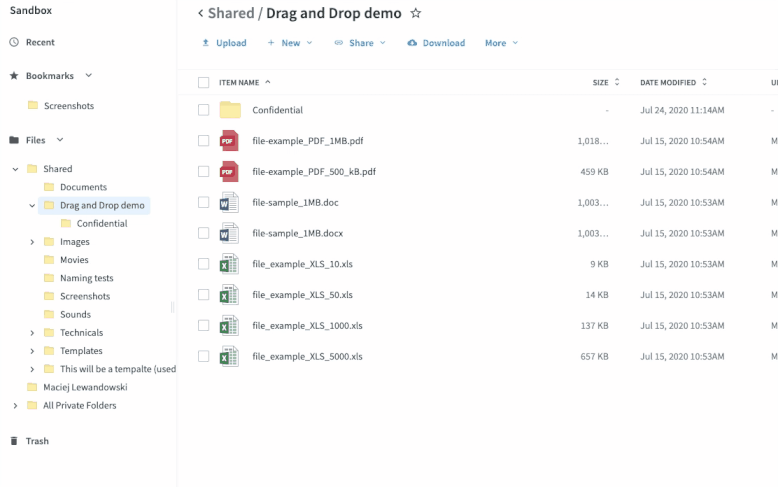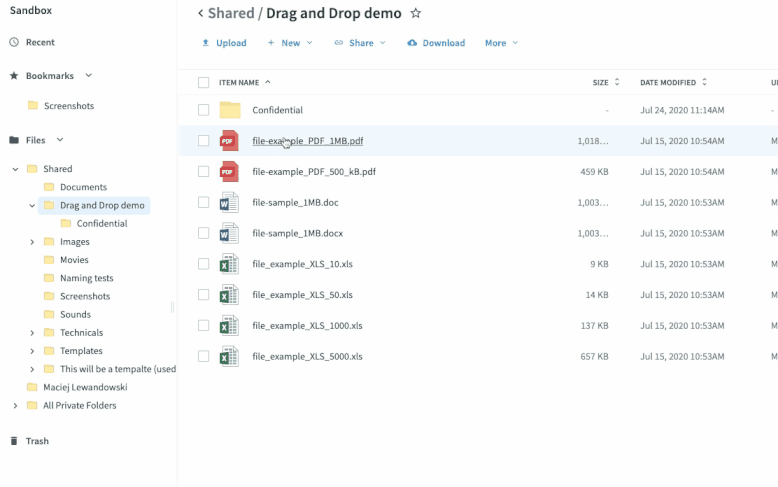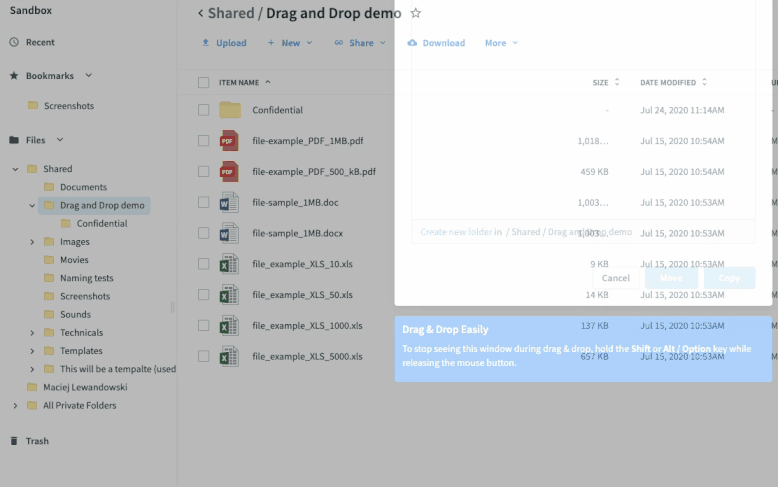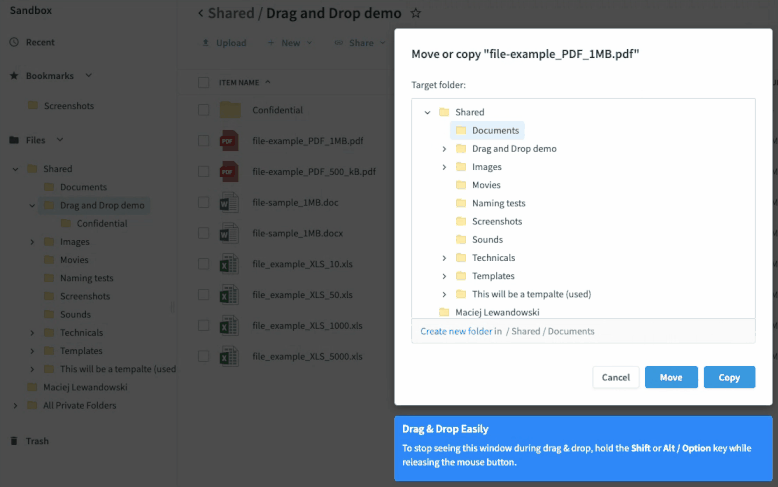
Once you’ve set up your submission process, sites take digitized source data and drag-and-drop it into the relevant folders. The process functions just like it does in your local file system folders—no new tools to learn or logins to remember. This has multiple benefits.
- It’s easy to adhere to each site’s workflow since the files are treated like any other file they digitize and store.
- The data is synced in seconds to the sponsor’s eTMF, accelerating the time from data collection to eTMF deployment.
- There is no extra login or training to be proficient. If you know how to save a file to a folder, you know how to use Egnyte. All the syncing and permissions happen in the cloud, making it easy to deploy and maintain compliance.
Add Integrations for Greater Automation
New or changed source files are synchronized to the sponsor’s system on the cloud, where it can trigger several actions.
First, an audit trail is created, marking who uploaded the file and timestamping it, complying with Part 11 requirements automatically.
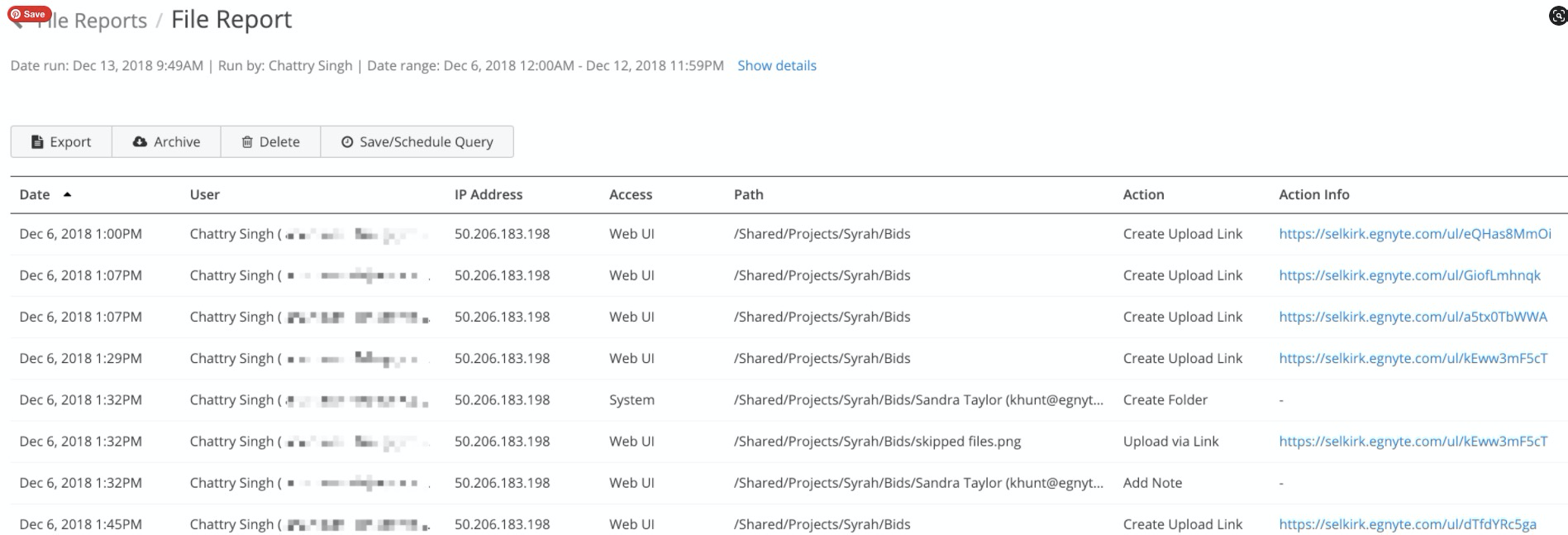
Next, a host of integrations can be triggered, from communication tools like Slack to ticketing systems like Jira to productivity tools like Microsoft 365.
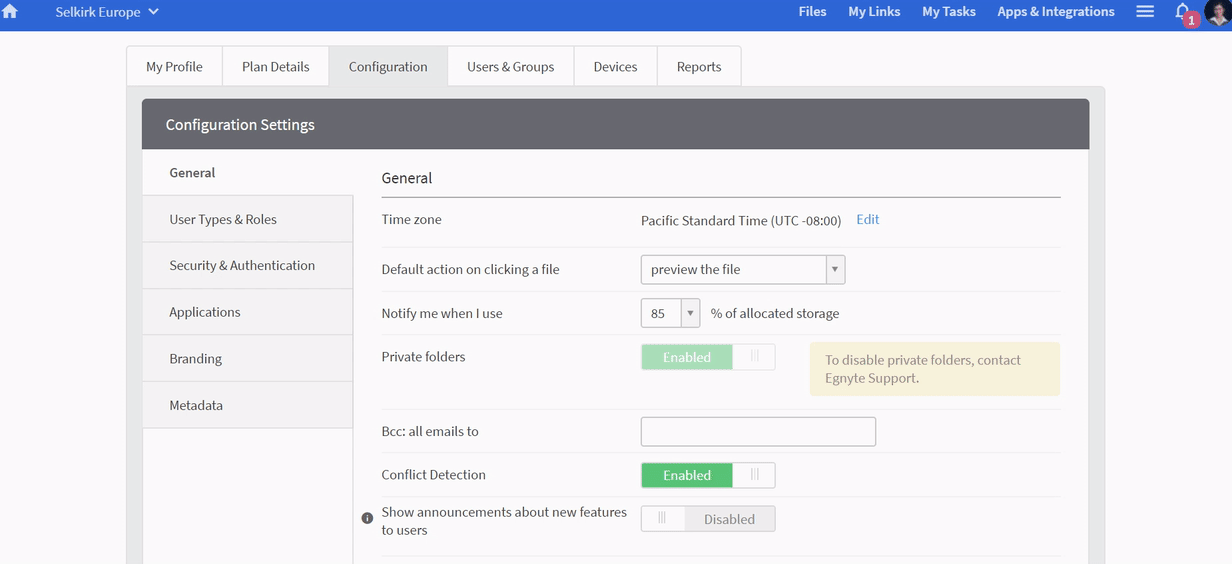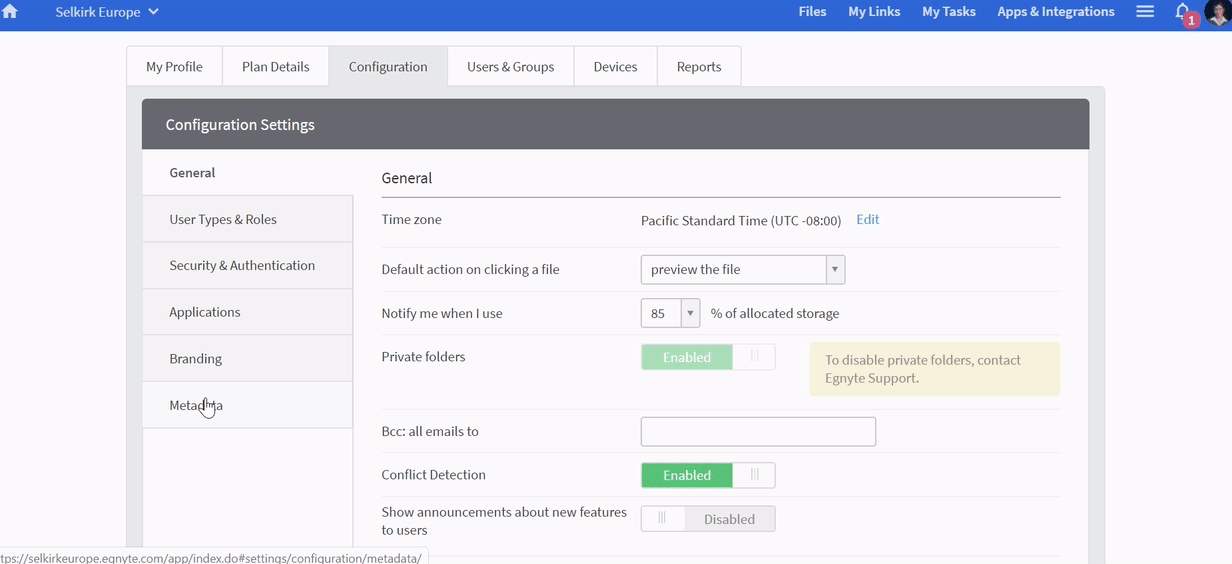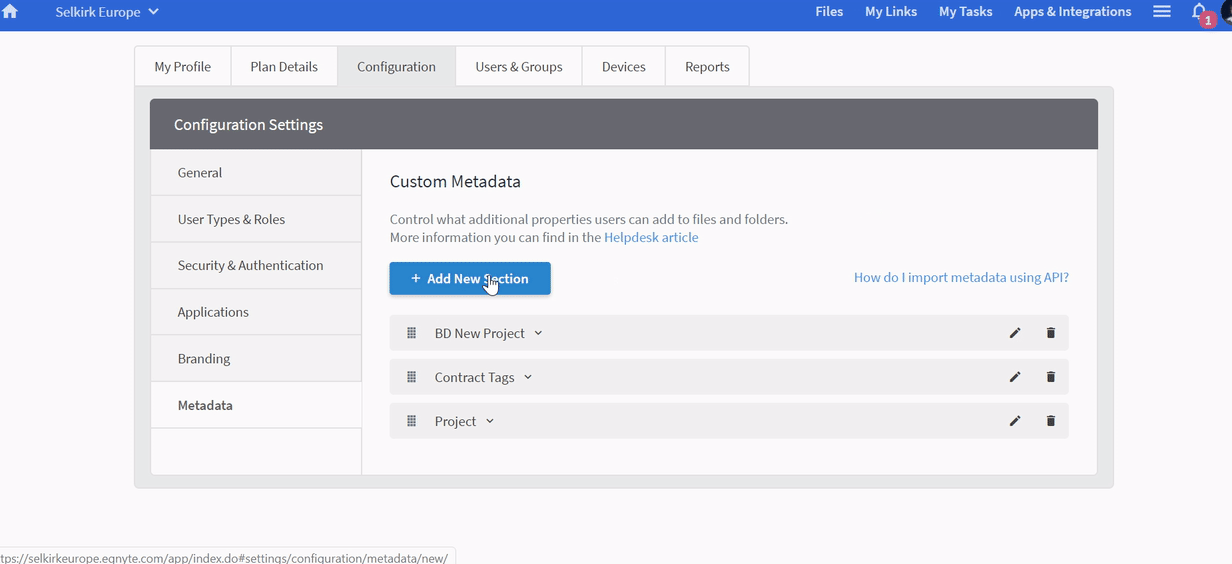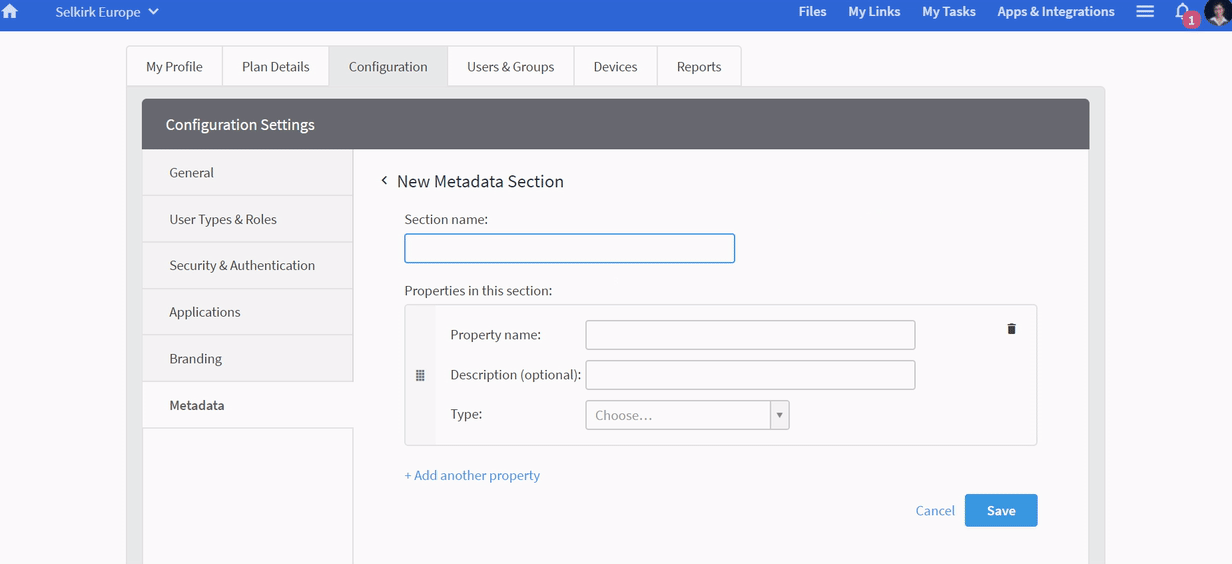
Egnyte will review the document and compare it against similar documents to automatically classify and flag misclassified or sensitive information as well as alert monitors to unexpected submissions.
.png)
You can have metadata added automatically for better tracking, and documents can be automatically cleaned up for contrast and sharpness to improve legibility before OCR-ing the document for search indexing.
These actions take place after the sites create source files, but they still improve the site submission process because the automation improvements:
- Reduce the administrative burden
- Improve data quality and integrity
- Reduce the time to deploy datasets into the eTMF from weeks to minutes
For nimble biotechs operating with a lean crew, being able to review the submission of source documentation faster has the knock-on effect of closing studies sooner.
Get Better Control of Your Trial Data
To reinforce your process and maximize your throughput, you must first identify where there is complexity and drag in your system. Operationalizing your data workflows improves data quality and integrity so you can be more confident in the insights that the data supports. Automating the workflows improves compliance and reduces the administrative burden of menial tasks.
And if these workflows can help support more fulfilling careers for the site staff, CRAs, and ClinOps teams, we all can feel better about the jobs we are doing in getting treatments to those who really need them.
To learn more on this topic, download Egnyte's guide to eTMFs to read about best practices around this cutting-edge technology.