Unlocking Faster Drug Discovery with Streamlined Metadata
The Life Sciences industry relies heavily on navigating massive volumes of data securely and efficiently to advance drug development and fuel new innovations in Research & Development (R&D). However, decentralized data and inconsistent metadata classification can hinder scientists from locating information, verifying quality, and reproducing findings, thereby, slowing down drug development timelines.
Challenge
R&D teams in the life sciences partner ecosystem often need help with similar data management issues. These issues include storing data in separate locations, outdated metadata standards, and incomplete or inaccurate contextual descriptors. This lack of consistency and accuracy in data classification can lead to scientists wasting a significant amount of time piecing together insights from disconnected systems. Therefore, it is crucial to have a unified approach to clinical data management to ensure data quality, relevance, and integrity.
Solution
Egnyte’s collaborative data platform helps leading life sciences organizations align around unified metadata standards. Our embedded data governance tools provide automated metadata capture and enforcement capabilities that standardize meanings and make data FAIR (Findable, Accessible, Interoperable, and Reusable). Researchers can easily search and discover datasets by their core attributes to accelerate drug development timelines.
Let’s Explore Key Capabilities
Egnyte’s platform enables seamless metadata management with:
- Intelligent metadata tagging to capture core descriptors like date, owner, instrument, and sample upon upload or edit.
- Trainable and controlled metadata classifiers that standardize terms and enforce validated values from drop-downs.
- Customizable metadata templates to control capture by document types.
- Dashboards tracking tagging coverage across projects.
Combined with AI-based data discovery, R&D teams can now uncover connections in data through a relevant metadata search instead of just keywords.
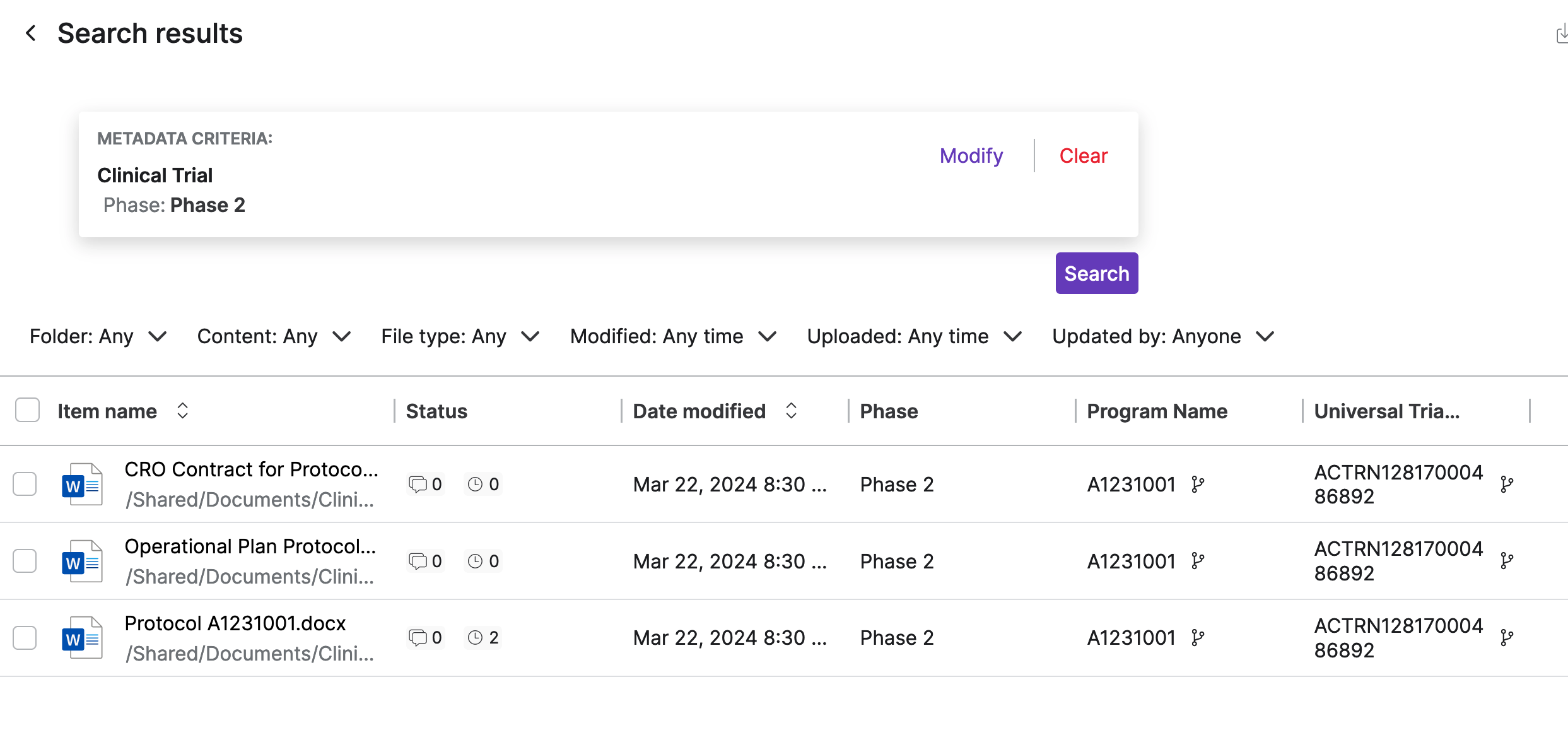
Egnyte enables users to write metadata directly into asset properties, ensuring that even if research data is moved out of Egnyte, the descriptive metadata remains, enabling downstream systems to detect IP status based on Egnyte’s advanced classifications and safeguard sensitive data.
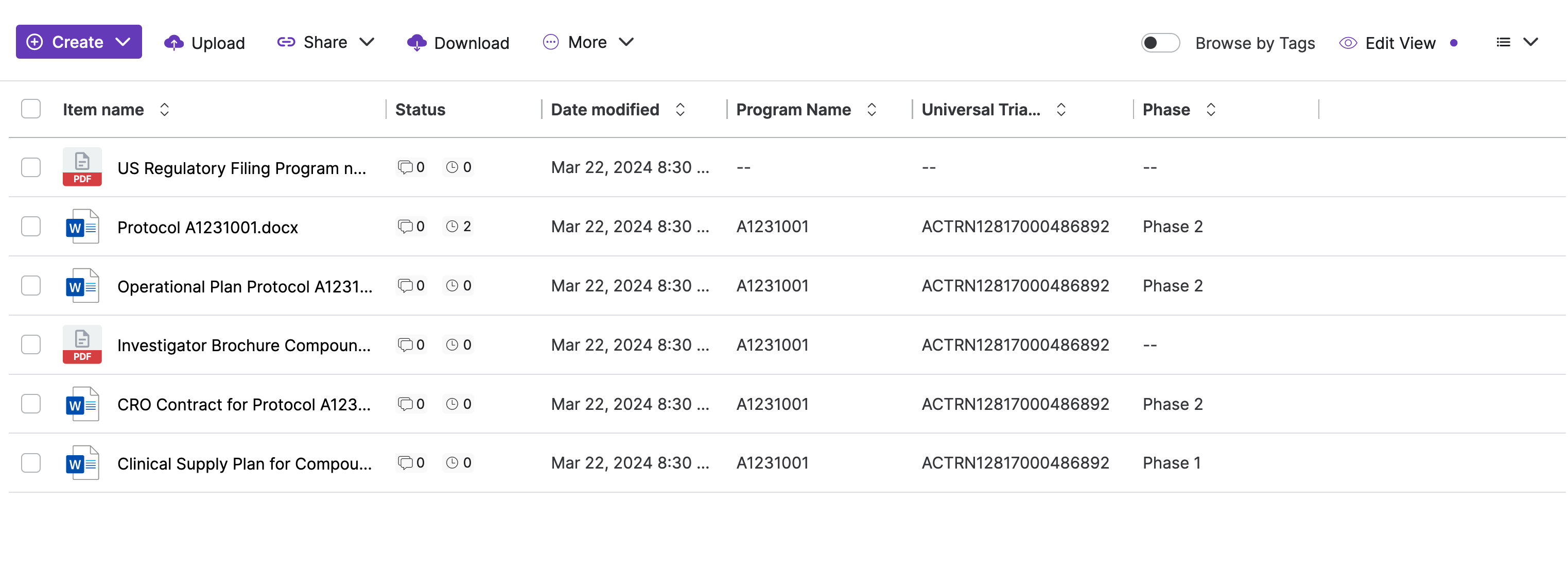
Key Benefits
With Egnyte’s metadata-driven detection, life sciences organizations can now:
- Accelerate trial milestones through IP visibility.
- Reduce data security risk by identifying sensitive assets.
- Simplify regulatory compliance via asset traceability.
- Optimize data FAIRness, improving accessibility and reuse.
By aligning metadata strategies, life sciences companies can now increase R&D productivity, accelerate time-to-market for new therapies, and ultimately improve patient outcomes. Egnyte enables life sciences companies to maximize value from research data by connecting meaning across tools, teams, and translational milestones.
Conclusion
Egnyte is committed to enhancing data classification tools with latest innovations and scalability, making it easier for life sciences users to manage their data. If you'd like to learn about Egnyte's data and metadata classification capabilities, please contact your Egnyte representative.