Ensuring Inspection Readiness With Egnyte’s eTMF QC Capability
Ensuring Inspection Readiness With Egnyte’s eTMF QC Capability
The life sciences industry is one of the most heavily regulated sectors that faces frequent inspections. As such, biotechs must implement rigorous quality control (QC) procedures to verify trial master file (TMF) documents meet stringent ALCOA+ standards. However, manual QC processes are time-consuming, prone to human error, and make maintaining continuous inspection readiness difficult as studies progress.
What is ALCOA+?
ALCOA+ provides a widely adopted framework for assessing TMF document quality in life sciences:
- Attributable – Authorship is definitively established.
- Legible – Content is readable and unambiguous.
- Contemporaneous – Records are created at the time of activity.
- Original – Content is initial capture and not a duplicate.
- Accurate – Information is factually correct.
- Available – Records are accessible when needed.
- Enduring – Content is fixed and unalterable.
- Complete – Records are comprehensive and final.
- Consistent – Uniform format and state-wide adherence.
Some common QC failures related to ALCOA+ requirements include:
- Missing or incorrect document dates.
- Illegible text or incorrect file versions.
- Inaccessible records due to access/permissions issues.
- Incomplete or inconsistent metadata.
- Alterations not tracked properly.
Challenge
While ALCOA+ provides a clear blueprint for assessing document quality, executing manual quality control processes presents difficulties for many biopharmas. As portfolios rapidly expand, these manual methods become time-intensive, prone to human error from repetitive tasks, and make maintaining continuous inspection readiness burdensome. This leads to inconsistent QC, delayed issue resolution, and scramble right before audits rather than systematic readiness.
Solution
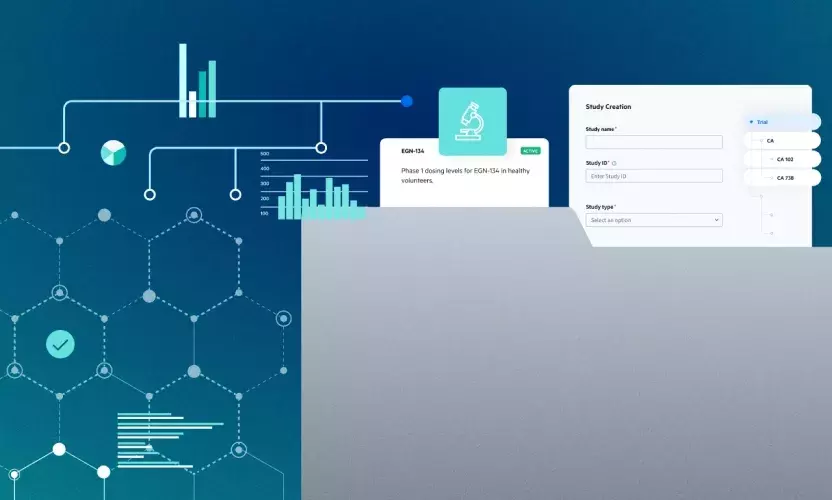
Egnyte has developed a built-in QC solution to ensure adherence to ALCOA+ principles. This new functionality enables biotechs to perform document quality reviews, accelerate approval cycles, and remain compliant as clinical trials progress.
How Egnyte Enables Efficient QC
Egnyte’s new eTMF QC feature allows sponsors to perform multiple document quality review processes such as:
- Automated QC task assignment with email notifications.
- Visual dashboard for review and approval status.
- Consolidated audit trail of all QC activity.

Conclusion
With Egnyte’s eTMF QC capabilities, life sciences organizations can perform document quality reviews tailored to specific study requirements. This enables continuous inspection readiness and compliance while optimizing resources allocated to document QC.
Schedule a demo today to learn how Egnyte’s purpose-built eTMF solution can take the complexity out of quality control within your organization while ensuring programs efficiently meet stringent regulatory demands.