Collaboration in the Modern Biotech Era
The life sciences industry has rapidly changed over the past twenty years. Drug development, historically, used to rely on vertically-integrated institutions that spanned scientific functions. However, today, products are launched by emerging startups, vast companies and any size in between. To improve velocity and agility, research is increasingly outsourced to academia and external partnerships beyond the traditional confines of a company. For example, some companies have 3-5 external scientists for every one internal researcher. The embrace of these external partnerships has made the life sciences industry more distributed, networked, and collaborative than ever before.
Scientific Service Providers
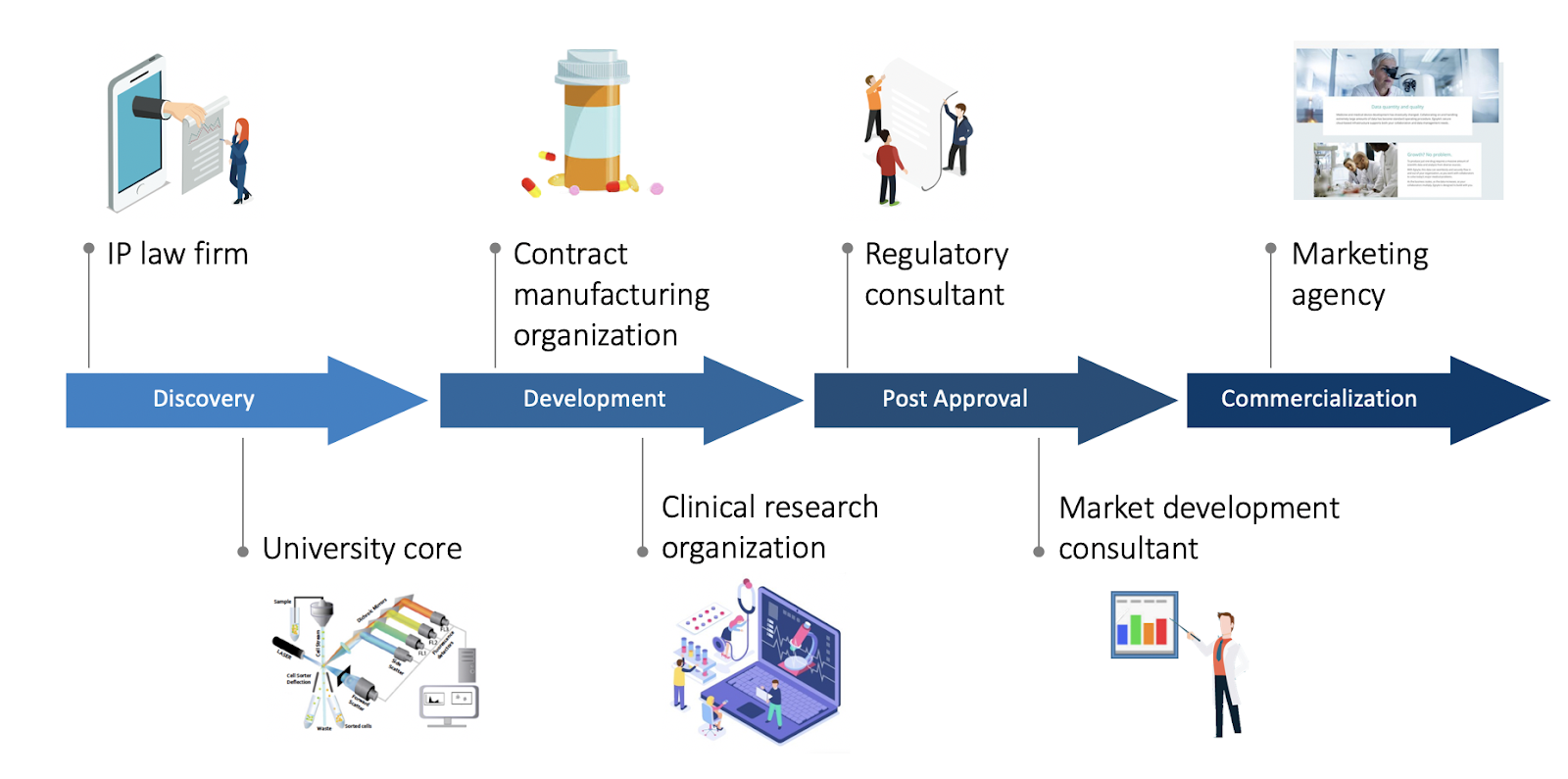
The Collaborators
When a company seeks external resources to complete a project, this is where the Contract Research Organization (CRO) can enter into the equation. A life sciences company can contract out parts of, or the entire development process. There are niche CROs that work solely in one area, like assay development or GLP toxicology, as well as larger scale CROs that can coordinate multiple aspects of clinical-stage testing and obtaining FDA approval, for example. Another subset of scientific services providers include Contract Manufacturing Organizations (CMO) or Contract Development and Manufacturing Organizations (CDMO) which can bridge the gap between research and manufacturing. Their services can include studies on scalability, stability, or manufacture of medicines themselves. The CRO market is expected to increase by 12% through 2021, leading to a projected global market of $44.4 billion in 2021. With companies of diverse sizes and capabilities trying to bring products to market, CROs and CMOs are serving a variety of needs within the life sciences community.
Academics
Outside of service providers, academic institutions are a prominent collaborator in the life sciences. Many research professors have spun out projects from the laboratory and into a startup, for example. Research universities also have resources, like core facilities and specialized instruments, that can be used by companies on-demand. University-based incubators have further blurred the line between industry startups and research laboratories, further cementing the University’s place as both a source and supporter of new companies.
Consultants
Beyond the traditional, laboratory-based collaborations of CROs and universities, there are other external individuals involved with drug development. For example, consultants take on roles in regulatory, market access, and quality in the early stages of a biotech. Depending on the area of expertise, they might not be performing laboratory experiments, but they are still compiling vital information that needs to be shared with the leading company. In the case of regulatory consultants, these experts will likely collaborate with multiple functions across the company, such as clinical and manufacturing.
Dynamic & Complex Collaboration
The collaborative ecosystem required to advance a compound can be expansive. At one end, a single asset in pre-clinical development can have half-a-dozen collaborators with expenditures exceeding $1M/year. On the other, a large pharmaceutical company can have over 800 external collaborators supporting their full pipeline. Due to the quantity of collaborations, and their different areas of involvement, this ecosystem of participants is becoming increasingly dynamic and complex. This environment requires that the sponsor have open lines of communication with each individual collaborator, but also coordinate the broader program’s progress and timelines. For example, starting a clinical trial is predicated upon receipt of drug product manufactured by a CMO. Further, to handle the evolving needs of projects, biopharma needs to be agile with collaborations, spinning them up and down as required.
Data is a big challenge
Managing the velocity and plethora of data produced by these dynamic collaborations is an increasingly prominent problem. A single drug program, for example, could produce data as varied as assays, pK/PD, and chemical structures. New high-throughput techniques for screening, imaging, and genomics only increases the quantity of data and exacerbates the challenges already present.
Current strategies for exchanging data between various contributors are insufficient for the life science community. Instead of tools designed to enable seamless collaborations, researchers are turning to poorly-functioning quick fixes: emails with data as attachments, powerpoints with screenshots, to name a few. At present, critical intellectual property or regulated content be lost or unintentionally shared, by accident, with the click of a button. Further, the fidelity of shared data is often limited to small sizes (e.g. 20 MB) or whatever can be embedded on a Powerpoint slide. Lastly, ‘shadow IT’ emerges where personal cloud storage, providing fewer restrictions on data type and size, are employed, violating company rules. All of these systems place the burden on the employee/researcher to share and retrieve data in arcane and inefficient ways. Such tools are poorly suited to the dynamic and real-time collaborations that characterize the life sciences.
Solutions
The life sciences community needs seamless solutions. The collaboration requirements of this community span countries and continents, and yet they must be able to engage as if they are next door. Tools need to deliver more value than effort put in. Ideally, software should enable three capabilities: i) agile communication and coordination, ii) effortless data transfer, and iii) data security.
Examples of solutions being used today include:
- Cloud Electronic Lab Notebooks (ELN)
- Secure Content Platforms (e.g. Egnyte)
- Firewalls/security Netskope
- Project management software (e.g. Smartsheets, SharePoint, etc.)
To find out more about secure content platforms for life sciences, check out www.egnyte.com