Why Life Sciences Needs the Science of Security
Those who have worked in the life sciences industry have undoubtedly observed a sea of change in the discipline over the past twenty years. From new modalities (like CAR-T to microbiome) to external collaborations, the way drugs are developed in the 21st century is more complex, more distributed, and faster. Alongside fundamentally new discoveries is a pan-industry shift from on-premise computing to the cloud. As this disruption occurs, the nuances of the life sciences industry results in a unique set of challenges as it pertains to security.
Data security is a serious topic that has reached public visibility on a near-daily basis. The life sciences industry, like other industries, is similarly concerned about data security and exposure of their data to malicious actors. A greater issue is one of intellectual property; as biopharmaceutical companies develop new treatments to disease, unplanned publication of their findings prior to patenting could result in substantial financial loss. Given the complexities that arise when securing data in drug development and developing software that facilitates common workflows, ensuring best-of-breed security simultaneously is the key to success. The three proven ways to securely advance science are:
- Collaboration with role-based access
- Ransomware protection
- Integrated compliance
Collaboration with role-based access
In many roles within drug development, from clinical operations to commercial supply to lead optimization, the phrase ‘firewalling’ is common parlance. This phrase, however, is rarely used to describe the IP networking infrastructure, but rather, the need to sequester pieces of data from various collaborators to ensure no single party (other than the sponsor) has full view of a study. The need to firewall is a challenge, as the work done with manufacturing, clinical and scientific partners are highly dynamic and international. To address this challenge, use a content collaboration software that can provide some role-based administrative rights to research project sponsors—with the ability to adjust permissions to ensure the right researchers have access to the right data.
Ransomware protection
In the news, we’ve seen numerous examples of cyberattacks crippling healthcare and life sciences focused companies. Some public examples are the Merck, Bayer, and Hancock Health cyberattacks. The unfortunate fact of cybersecurity is that as data, collaborations, and devices expand, the possible avenues for misappropriation of data is greater than ever. Consider implementing a strategy for data security that enables stakeholders to protect critical assets from malicious attacks.
Integrated compliance
In addition to fretting about security, many data-driven organizations worry about an ever-evolving world of regulations. Compliance with data privacy, data residency, among other policies creates an evolving set of challenges for the modern life sciences organization. Combining collaboration capability with compliance protocols is ideal. Two common uses for a platform like this is to retrofit existing data repositories with data archiving and policy-driven data management. In the case of the former, the FDA requires data to be archived, while maintaining data integrity, for lengthy periods of time. Automating this process would enable the user to set data retention policies of customized length to ensure compliance. Further, as the European Union and other locales regulate the use of Personally Identifiable Information (PII), business stakeholders and IT groups must comply with GDPR-like rules. Organizations can comply with GDPR by locating, controlling and securing the PII of EU residents stored in on-premises or cloud repositories. Look for software that offers these capabilities built-in.
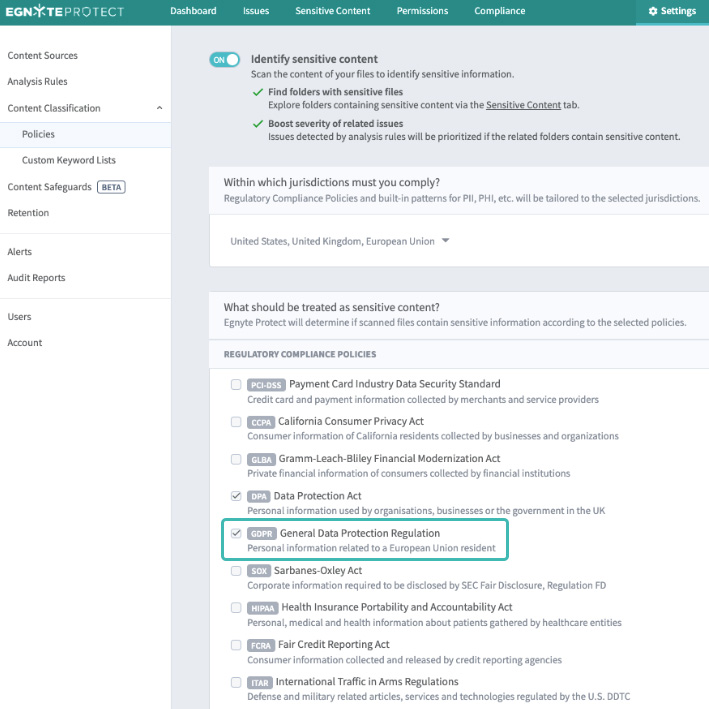
As drug development evolves, everyone in biopharma is paranoid about the impact of data security on their work. For stakeholders, from scientists to IT, these assets must be protected. With conventional file sharing tools, a major challenge is the sacrifice users make to enable security. This doesn’t have to be the case. Egnyte’s platform provides seamless collaboration alongside world-class security through policy automation and application across existing repositories.
Learn more about what Egnyte is doing for its life sciences customers.